Phase transited and vapor-induced dual capsular system (DCS) for achieving delayed and osmotic release of cefadroxil.
Anil K Philip, Betty Philip
Index: Pharm. Dev. Technol. 16(5) , 457-65, (2011)
Full Text: HTML
Abstract
In the present study, an intestinal pH, disintegrating and non-disintegrating dual capsular system using formaldehyde vapor and phase transition technique, respectively, was developed to achieve delayed as well as improved osmotic flow for the model drug cefadroxil. Formaldehyde vapor was used to attain gastric resistance to the outer gelatin capsule, which disintegrated at the intestinal pH to give a non-disintegrating asymmetric membrane capsule (AMC). The AMC was prepared via dry phase inversion process. The effects of different formulation variables were studied based on 2³ factorial design, namely, level of osmogen, ethylcellulose, and pore former, apart from studying the effects of varying osmotic pressure, agitation intensity, and intentional defect on drug release. Scanning electron microscopy showed an outer dense non-porous and an inner lighter porous region for the prepared asymmetric membrane. Statistical test was applied for in-vitro drug release at P > 0.05. The best formulation in the design closely corresponded to the extra design checkpoint formulation by a similarity (f₂) value of 95.28. The drug release was independent of the agitation intensity and intentional defect of the film but dependent on the osmotic pressure of the dissolution medium. The release kinetics followed zero-order, and mechanism of release was Fickian diffusion.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
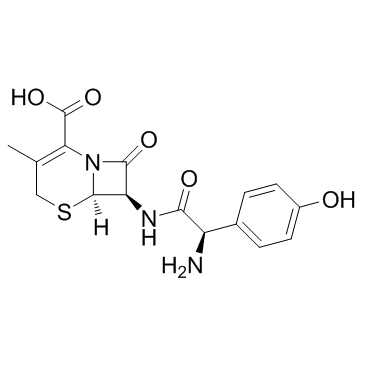 |
Cefadroxil
CAS:50370-12-2 |
C16H17N3O5S |
|
Cefadroxil. A review of its antibacterial, pharmacokinetic a...
1986-01-01 [Drugs 32 Suppl 3 , 1-16, (1986)] |
|
Solubility-pH profiles of some acidic, basic and amphoteric ...
2013-01-23 [Eur. J. Pharm. Sci. 48(1-2) , 291-300, (2013)] |
|
Study on the binding behavior of bovine serum albumin with c...
2010-12-15 [Talanta 83(2) , 312-9, (2010)] |
|
Fluorescence studies of the dehydration of cefadroxil monohy...
2007-10-01 [J. Pharm. Sci. 96(10) , 2757-64, (2007)] |
|
Bioequivalence study of two oral formulations of cefadroxil ...
2008-01-01 [Arzneimittelforschung 58(1) , 42-7, (2008)] |