Pharmacokinetics of luxabendazole after oral and intravenous administration to sheep.
A I Ortiz, L Alvarez-Bujidos, I Ferre, D Ordóñez
Index: Am. J. Vet. Res. 58(11) , 1263-6, (1997)
Full Text: HTML
Abstract
To determine pharmacokinetics of luxabendazole after oral and i.v. administration to healthy sheep.7 clinically normal female Merino sheep between 9 and 12 months old.Pharmacokinetics were determined after oral and i.v. administration of luxabendazole at a dose of 10 mg/kg of body weight. Serial blood samples were collected for 56 hours after administration. Plasma concentrations of luxabendazole were determined by high-pressure liquid chromatography.After i.v. administration, elimination of luxabendazole was slow, with a mean half-life of 8.72 hours. Mean steady-state volume of distribution and mean distribution volume during the elimination phase were 3.18 and 3.10 L/kg, respectively. Mean clearance was 0.24 L/kg.h, and mean area under the concentration-time curve was 41.89 mg.h/L. After oral administration, luxabendazole was slowly absorbed from the gastrointestinal tract. Mean absorption half-life was 2.26 hours. Peak plasma concentration was 0.50 microgram/ml and was detected 14 to 16 hours after drug administration. Mean area under the concentration-time curve was 12.03 mg.h/L. Mean bioavailability was 29%.Results suggest that luxabendazole is moderately absorbed from the gastrointestinal tract in sheep, is widely distributed into extravascular compartments, and is cleared slowly.Determination of pharmacokinetic parameters is the first step in determining a safe and efficacious dosage regimen for luxabendazole in sheep.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
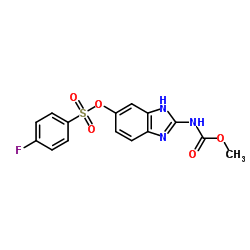 |
Luxalbendazole
CAS:90509-02-7 |
C15H12FN3O5S |
|
Activity of luxabendazole against liver flukes, gastrointest...
1988-01-01 [Parasitol. Res. 75(1) , 14-8, (1988)] |
|
Determination of luxabendazole in biological fluids by high-...
[J. Chromatogr. A. 578(2) , 321-6, (1992)] |
|
Biochemical effects of luxabendazole on Trichinella spiralis...
1990-01-01 [Parasitol. Res. 76(6) , 518-20, (1990)] |
|
Bacterial mutagenic evaluation of Luxabendazole, a new broad...
1996-01-01 [Mutagenesis 11(1) , 27-31, (1996)] |
|
The intestinal absorption of Luxabendazole in rats.
1994-11-01 [J. Pharm. Biomed. Anal. 12(11) , 1471-4, (1994)] |