| Structure | Name/CAS No. | Articles |
|---|---|---|
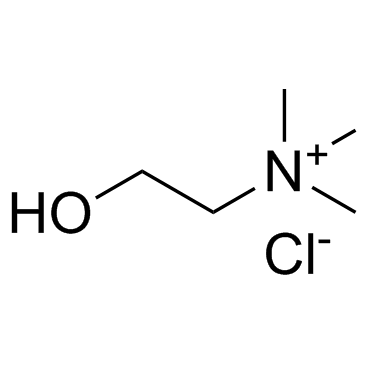 |
Choline chloride
CAS:67-48-1 |
|
 |
Glycerophosphocholine phosphodiesterase
CAS:9025-85-8 |