The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade.
Camille Bonomelli, Katie J Doores, D Cameron Dunlop, Victoria Thaney, Raymond A Dwek, Dennis R Burton, Max Crispin, Christopher N Scanlan
Index: PLoS ONE 6 , e23521, (2011)
Full Text: HTML
Abstract
The N-linked oligomannose glycans of HIV gp120 are a target for both microbicide and vaccine design. The extent of cross-clade conservation of HIV oligomannose glycans is therefore a critical consideration for the development of HIV prophylaxes. We measured the oligomannose content of virion-associated gp120 from primary virus from PBMCs for a range of viral isolates and showed cross-clade elevation (62-79%) of these glycans relative to recombinant, monomeric gp120 (∼30%). We also confirmed that pseudoviral production systems can give rise to notably elevated gp120 oligomannose levels (∼98%), compared to gp120 derived from a single-plasmid viral system using the HIV(LAI) backbone (56%). This study highlights differences in glycosylation between virion-associated and recombinant gp120.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
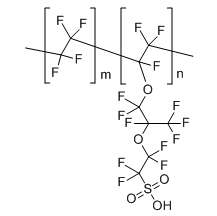 |
nafion
CAS:31175-20-9 |
(C7HF13O5S.C2F4)x |
|
Conversion of polar and non-polar algae oil lipids to fatty ...
2015-09-01 [Bioresour. Technol. 191 , 300-5, (2015)] |
|
Flexible Lamination Encapsulation.
2015-08-05 [Adv. Mater. 27 , 4308-14, (2015)] |
|
Development of a low-cost optical sensor for cupric reducing...
2010-05-15 [Anal. Chem. 82(10) , 4252-8, (2010)] |
|
[J. Electrochem. Soc. 140 , L55-57, (1993)] |
|
Pt-Ir-IrO2NT thin-wall electrocatalysts derived from IrO2 na...
[Chem. Mater. 19(3) , 424-431., (2007)] |