| Structure | Name/CAS No. | Articles |
|---|---|---|
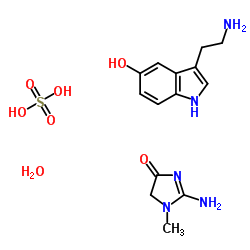 |
SEROTONIN CREATININE SULFATE MONOHYDRATE
CAS:61-47-2 |
|
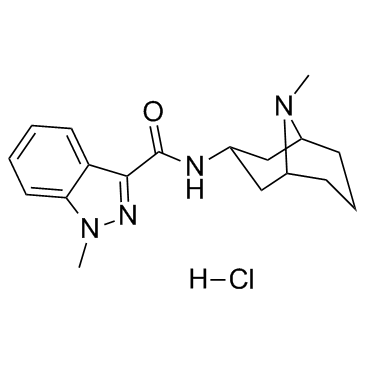 |
Granisetron hydrochloride
CAS:107007-99-8 |
|
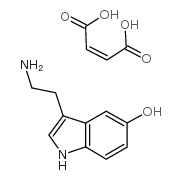 |
Serotonin hydrogen maleate
CAS:18525-25-2 |
|
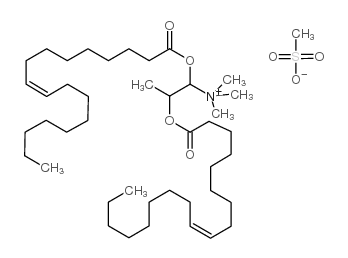 |
DOTAP Transfection Reagent
CAS:144189-73-1 |
|
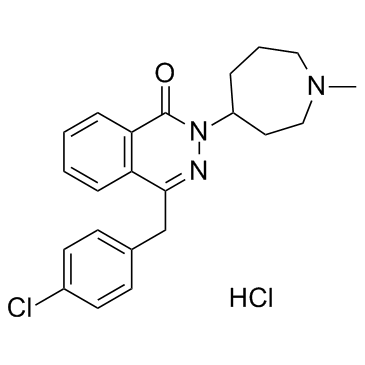 |
azelastine hydrochloride
CAS:79307-93-0 |