A Second-generation photocage for Zn2+ inspired by TPEN: characterization and insight into the uncaging quantum yields of ZinCleav chelators.
H M Dhammika Bandara, Timothy P Walsh, Shawn C Burdette
Index: Chemistry 17(14) , 3932-41, (2011)
Full Text: HTML
Abstract
Photocages have been used to elucidate the biological functions of various small molecules and Ca(2+) ; however, there are very few photocages available for other metal ions. ZinCleav-2 (1-(4,5-dimethoxy-2-nitrophenyl)-N,N,N',N'-tetrakis-pyridin-2-ylmethyl-ethane-1,2-diamine) is a second-generation photocage for Zn(2+) that releases the metal ion after a light-induced bifurcation of the chelating ligand. The structure of ZinCleav-2 was inspired by TPEN (N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine), which is routinely used to sequester metal ions in cells owing to its high binding affinity. Inclusion of a 2-nitrobenzyl chromophore leads to the formation of two more weakly binding di-(2-picolyl)amine (DPA) fragments upon photolysis of the TPEN backbone. The desired ligand was prepared using a modified procedure used to access ZinCleav-1 (1-(4,5-dimethoxy-2-nitrophenyl)-N,N'-dimethyl-N,N'-bis-pyridin-2-ylmethyl-ethane-1,2-diamine). ZinCleav-2 has a conditional dissociation constant (K(d) ) of ∼0.9 fM as measured by competitive titration with a quinoline-based fluorescent sensor for Zn(2+). The K(d) of the Zn(2+) complex of the DPA photoproducts is ∼158 nM; therefore, the ΔK(d) for ZinCleav-2 photocage is ∼10(8). A large ΔK(d) is required to significantly perturb free metal ion concentrations in biological assays. The quantum yield of photolysis of apo ZinCleav-2 and the [Zn(ZinCleav-2)](2+) complex are 4.7 and 2.3 %, respectively, as determined by HPLC analysis. Proof of concept Zn(2+) release upon photolysis of [Zn(ZinCleav-2)](2+) was demonstrated using the fluorescent sensor Zinpyr-1, and the speciation of Zn(2+) complexes was simulated using computational methods. The influence of benzylic substituents on the quantum yield of uncaging is also analyzed with the aim of tuning the photochemical properties caged complexes for in vivo experiments.Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
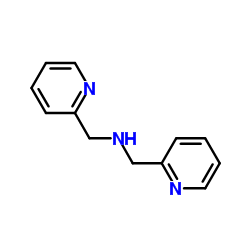 |
Di-(2-picolyl)amine
CAS:1539-42-0 |
C12H13N3 |
|
Identifying chelators for metalloprotein inhibitors using a ...
2011-01-27 [J. Med. Chem. 54 , 591-602, (2011)] |
|
Efficient hydrolytic cleavage of plasmid DNA by chloro-cobal...
2014-07-14 [Dalton Trans. 43(26) , 10086-103, (2014)] |
|
Formation of a metal-to-nitrogen bond of normal length by a ...
2013-03-04 [Inorg. Chem. 52(5) , 2412-21, (2013)] |
|
Spin crossover and polymorphism in a family of 1,2-bis(4-pyr...
2011-10-07 [Dalton Trans. 40(37) , 9608-18, (2011)] |
|
Metal complexes with functionalised 2,2'-dipicolylamine liga...
2013-05-21 [Dalton Trans. 42(19) , 6817-28, (2013)] |