Spin crossover and polymorphism in a family of 1,2-bis(4-pyridyl)ethene-bridged binuclear iron(II) complexes. A key role of structural distortions.
Galina S Matouzenko, Erwann Jeanneau, Alexander Yu Verat, Azzedine Bousseksou
Index: Dalton Trans. 40(37) , 9608-18, (2011)
Full Text: HTML
Abstract
Two polymorphic modifications 1 and 3 of binuclear compound [{Fe(dpia)(NCS)(2)}(2)(bpe)] and pseudo-polymorphic modification [{Fe(dpia)(NCS)(2)}(2)(bpe)]·2CH(3)OH (2), where dpia = di-(2-picolyl)amine, bpe = 1,2-bis(4-pyridyl)ethene, were synthesized, and their structures, magnetic properties, and Mössbauer spectra were studied. Variable-temperature magnetic susceptibility measurements of three binuclear compounds show different types of magnetic behaviour. The complex 1 exhibits a gradual two-step spin crossover (SCO) suggesting the occurrence of the mixed [HS-LS] (HS: high spin, LS: low spin) pair at the plateau temperature (182 K), at which about 50% of the complexes undergoes a thermal spin conversion. The complex 2 displays an abrupt full one-step spin transition without hysteresis, centred at about 159 K. The complex 3 is paramagnetic over the temperature range 20-290 K. The single-crystal X-ray studies show that all three compounds are built up from the bpe-bridged binuclear molecules. The structure of 1 was solved for three spin isomers [HS-HS], [HS-LS], and [LS-LS] at three temperatures 300 K, 183 K, and 90 K. The crystal structures for 2 and 3 were determined for the [HS-HS] complexes at room temperature. The analysis of correlations between the structural characteristics and different types of magnetic behaviour for new 1-3 binuclear complexes, as well as for previously reported binuclear compounds, revealed that the SCO process (occurrence of full one-step, two-step, or partial (50%) SCO) is specified by the degree of distortion of the octahedral geometry of the [FeN(6)] core, caused by both packing and strain effects arising from terminal and/or bridging ligands. The comparison of the magnetic properties and the networks of intra- and inter-molecular interactions in the crystal lattice for the family of related SCO binuclear compounds suggests that the intermolecular interactions play a predominant role in the cooperativeness of the spin transition relative to the intramolecular interactions through the bridging ligand.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
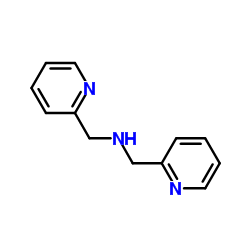 |
Di-(2-picolyl)amine
CAS:1539-42-0 |
C12H13N3 |
|
Identifying chelators for metalloprotein inhibitors using a ...
2011-01-27 [J. Med. Chem. 54 , 591-602, (2011)] |
|
Efficient hydrolytic cleavage of plasmid DNA by chloro-cobal...
2014-07-14 [Dalton Trans. 43(26) , 10086-103, (2014)] |
|
Formation of a metal-to-nitrogen bond of normal length by a ...
2013-03-04 [Inorg. Chem. 52(5) , 2412-21, (2013)] |
|
Metal complexes with functionalised 2,2'-dipicolylamine liga...
2013-05-21 [Dalton Trans. 42(19) , 6817-28, (2013)] |
|
A Second-generation photocage for Zn2+ inspired by TPEN: cha...
2011-03-28 [Chemistry 17(14) , 3932-41, (2011)] |