Regulation by 1,4-diamines of the ornithine decarboxylase activity induced by ornithine in perifused tumor cells.
J M Matés, F Sánchez-Jiménez, J López-Herrera, I Núñez de Castro
Index: Biochem. Pharmacol. 42(5) , 1045-52, (1991)
Full Text: HTML
Abstract
Ornithine decarboxylase (ODC) activity of Ehrlich carcinoma cells was increased more than 36-fold after being maintained for 3.5 hr in vitro in a special chamber which allowed continuous perifusion with 0.5 mM ornithine; if incubated in vitro without perifusion the ODC activity was, of course, only 9-fold by the same concentration of ornithine. Ornithine withdrawal from the perifusion medium resulted in a decay of enzyme activity observed after 90 min; this decay was prevented by addition of 55 microM pyridoxal to the medium. The 1,4-diamines putrescine, spermidine, spermine, agmatine, histamine, serotonin, tryptamine, chlorpheniramine and harmaline at 55 microM strongly suppressed ODC induction by 0.5 mM ornithine in perifused Ehrlich ascites cells. Methyl derivatives also behave as strong inhibitors of ODC induction. On the contrary, N-acetylation paralleled with a decrease in the inhibition capacity: 55 microM N-acetyl putrescine, N-acetyl serotonin or N-omega-acetylhistamine suppressed ODC induction by ornithine in 66, 64 and 19%, respectively. The addition to the perifusion medium of the same concentrations of 1,3-diamines (1,3-diaminopropane, 1,3-diamino-2-propanol or the alkaloid gramine) as well as 1,5-diamines (1,5-diaminopentane and the antihistamic doxylamine or cimetidine) failed to suppress the induction of ODC activity by ornithine. Interestingly, 1,4-benzenediamine, which strongly inhibits ODC activity when the induced enzyme is assayed in its presence, did not suppress the induction of the enzyme when both 0.5 mM ornithine and 55 microM 1,4-benzenediamine were present in the perifusion medium. The inhibitory capacity in down-regulating ODC is not due to differences in the diamine uptake by the cells. The results suggest that the N-N distance (6A) and the charge of one amino group are important chemical characteristics for regulatory effects.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
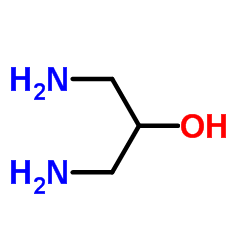 |
1,3-Diaminopropan-2-ol
CAS:616-29-5 |
C3H10N2O |
|
Mimicking peroxidase activity by a polymer-supported oxidova...
2015-06-01 [J. Inorg. Biochem. 147 , 181-92, (2015)] |
|
Amino alcohol-based degradable poly(ester amide) elastomers.
2008-05-01 [Biomaterials 29(15) , 2315-25, (2008)] |
|
Efficient gene transfection using novel cationic polymers po...
2010-09-15 [Bioconjug. Chem. 21(9) , 1602-11, (2010)] |
|
Copper(II) mediated anion dependent formation of Schiff base...
2005-05-30 [Inorg. Chem. 44(11) , 3880-9, (2005)] |
|
Regulation of S-adenosylmethionine decarboxylase by polyamin...
1980-09-15 [Biochem. J. 190(3) , 747-54, (1980)] |