Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture.
L Alhonen-Hongisto
Index: Biochem. J. 190(3) , 747-54, (1980)
Full Text: HTML
Abstract
1. The mechanism of stimulation of S-adenosylmethionine decarboxylase (EC 4.1.1.50) activity by inhibitors of ornithine decarboxylase (EC 4.1.1.17), namely dl-alpha-difluoromethylornithine, 1,3-diaminopropane and 1,3-diaminopropan-2-ol, was studied in Ehrlich ascites-tumour cells grown in suspension cultures. 2. Difluoromethylornithine and diaminopropane, although decreasing the content of putrescine and spermidine, markedly stimulated adenosylmethionine decarboxylase activity after exposure of the cells to the drugs for 8h, whereas the effect of diaminopropanol only became apparent many hours later. In tumour cells exposed to any of the inhibitors, a close negative correlation existed between the activity of adenosylmethionine decarboxylase and the intracellular concentration of spermidine and/or spermidine plus spermine, suggesting that a depletion of higher polyamines triggered enhancement of adenosylmethionine decarboxylase activity. 3. The mechanism of difluoromethylornithine- and diaminopropane-induced stimulation of adenosylmethionine decarboxylase involved (a) a marked increase in the apparent half-life of the enzyme and (b) an induction of enhanced enzyme synthesis. Diaminopropanol seemed to act solely via an induction mechanism. 4. The increased adenosylmethionine decarboxylase activity elicited by difluoromethylornithine could be restored to control values by micromolar concentrations of exogenous spermidine and spermine in 4h and by putrescine in 22h. In addition to the natural polyamines, elevated adenosylmethionine decarboxylase activity could be repressed by 3,3'-iminodipropylamine, a close analogue of spermidine, but not by non-physiological diamines. 5. Addition of spermidine and actinomycin D to cultures treated with difluoromethylornithine produced a comparable decay of enhanced adenosylmethionine decarboxylase activity (with an apparent half-life of about 2.5h), whereas the effect of cycloheximide was much more rapid. The present results suggest that polyamines may regulate adenosylmethionine decarboxylase at the transcriptional level of gene expression.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
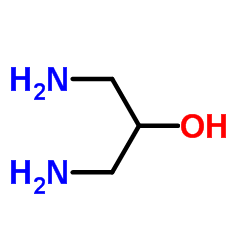 |
1,3-Diaminopropan-2-ol
CAS:616-29-5 |
C3H10N2O |
|
Mimicking peroxidase activity by a polymer-supported oxidova...
2015-06-01 [J. Inorg. Biochem. 147 , 181-92, (2015)] |
|
Amino alcohol-based degradable poly(ester amide) elastomers.
2008-05-01 [Biomaterials 29(15) , 2315-25, (2008)] |
|
Efficient gene transfection using novel cationic polymers po...
2010-09-15 [Bioconjug. Chem. 21(9) , 1602-11, (2010)] |
|
Copper(II) mediated anion dependent formation of Schiff base...
2005-05-30 [Inorg. Chem. 44(11) , 3880-9, (2005)] |
|
Inhibition by derivatives of diguanidines of cell proliferat...
1980-05-15 [Biochem. J. 188(2) , 491-501, (1980)] |