Activity of α-Aminoadipate Reductase Depends on the N-Terminally Extending Domain.
Daniel Kalb, Gerald Lackner, Marcus Rappe, Dirk Hoffmeister
Index: ChemBioChem. 16(10) , 1426-30, (2015)
Full Text: HTML
Abstract
L-α-Aminoadipic acid reductases catalyze the ATP- and NADPH-dependent reduction of L-α-aminoadipic acid to the corresponding 6-semialdehyde during fungal L-lysine biosynthesis. These reductases resemble peptide synthetases with regard to their multidomain composition but feature a unique domain of elusive function--now referred to as an adenylation activating (ADA) domain--that extends the reductase N-terminally. Truncated enzymes based on NPS3, the L-α-aminoadipic acid reductase of the basidiomycete Ceriporiopsis subvermispora, lacking the ADA domain either partially or entirely were tested for activity in vitro, together with an ADA-adenylation didomain and the ADA domainless adenylation domain. We provide evidence that the ADA domain is required for substrate adenylation: that is, the initial step of the catalytic turnover. Our biochemical data are supported by in silico modeling that identified the ADA domain as a partial peptide synthetase condensation domain.© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
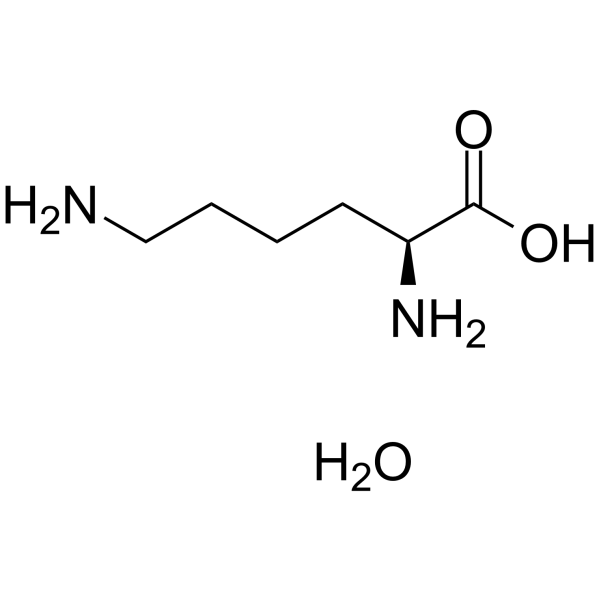 |
L(+)-Lysine monohydrate
CAS:39665-12-8 |
C6H16N2O3 |
|
Direct cellular delivery of human proteasomes to delay tau a...
2014-01-01 [Nat. Commun. 5 , 5633, (2014)] |
|
A new antitumor enzyme, L-lysine alpha-oxidase from Trichode...
1980-02-10 [J. Biol. Chem. 255 , 976, (1980)] |
|
Phosphoproteomic Profiling Reveals Epstein-Barr Virus Protei...
2015-12-01 [PLoS Pathog. 11(12) , e1005346, (2015)] |
|
Identification of inhibitors of chromatin modifying enzymes ...
2009-01-01 [Methods Mol. Biol. 548 , 145-60, (2009)] |
|
A practical recipe for stable isotope labeling by amino acid...
2006-01-01 [Nat. Protoc. 1(6) , 2650-60, (2006)] |