An efficient carbonyl-alkene metathesis of bicyclic oxetanes: photoinduced electron transfer reduction of the Paternò-Büchi adducts from 2,3-dihydrofuran and aromatic aldehydes.
Raúl Pérez-Ruiz, Miguel A Miranda, Ronald Alle, Klaus Meerholz, Axel G Griesbeck
Index: Photochem. Photobiol. Sci. 5(1) , 51-5, (2006)
Full Text: HTML
Abstract
The bicyclic oxetanes and resulting from photocycloaddition of aromatic aldehydes to 2,3-dihydrofuran, were efficiently cleaved by means of electron-transfer reduction, photoinduced by the electronically excited reductants 1-methoxynaphthalene (MN) and 2,7-dimethoxynaphthalene (DMN) in acetonitrile. The fluorescence quenching rates of DMN/MN by and were determined by static methods, the triplet quenching rates were determined by means of laser flash photolysis (LFP). The product analysis established a "photo-photo metathesis" where both cycloaddition and cycloreversion processes are induced by photochemical processes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
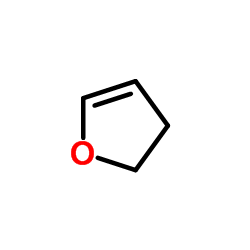 |
2,3-DHF
CAS:1191-99-7 |
C4H6O |
|
An integrated microreactor system for self-optimization of a...
2010-09-17 [Angew. Chem. Int. Ed. Engl. 49(39) , 7076-80, (2010)] |
|
A gold(I)-catalyzed intramolecular reaction of propargylic/h...
2008-01-01 [Chemistry 14(23) , 7011-8, (2008)] |
|
Short, enantioselective total synthesis of aflatoxin B2 usin...
2005-08-31 [J. Am. Chem. Soc. 127(34) , 11958-9, (2005)] |
|
Phosphine-catalyzed domino reaction for the synthesis of con...
2012-06-01 [Chem. Asian J. 7(7) , 1533-7, (2012)] |
|
First successful application of diphosphite ligands in the a...
2005-03-07 [Chem. Commun. (Camb.) (9) , 1221-3, (2005)] |