Metal complexes of Schiff base derived from sulphametrole and o-vanilin. Synthesis, spectral, thermal characterization and biological activity.
Gehad G Mohamed, Carmen M Sharaby
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 66(4-5) , 949-58, (2007)
Full Text: HTML
Abstract
Metal complexes of Schiff base derived from condensation of o-vanilin (3-methoxysalicylaldehyde) and sulfametrole [N(1)-(4-methoxy-1,2,5-thiadiazole-3-yl)sulfanilamide] (H2L) are reported and characterized based on elemental analyses, IR, 1H NMR, solid reflectance, magnetic moment, molar conductance, mass spectra, UV-vis and thermal analysis (TGA). From the elemental analyses data, the complexes were proposed to have the general formulae [M2X3(HL)(H2O)5].yH2O (where M=Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II), X=Cl, y=0-3); [Fe2Cl5(HL)(H2O)3].2H2O; [(FeSO4)2(H2L)(H2O)4] and [(UO2)2(NO3)3(HL)(H2O)].2H2O. The molar conductance data reveal that all the metal chelates were non-electrolytes. The IR spectra show that, H2L is coordinated to the metal ions in a tetradentate manner with ON and NO donor sites of the azomethine-N, phenolic-OH, enolic sulphonamide-OH and thiadiazole-N. From the magnetic and solid reflectance spectra, it is found that the geometrical structures of these complexes are octahedral. The thermal behaviour of these chelates shows that the hydrated complexes losses water molecules of hydration in the first step followed immediately by decomposition of the anions and ligand molecules in the subsequent steps. The activation thermodynamic parameters, such as, E*, DeltaH*, DeltaS* and DeltaG* are calculated from the DrTG curves using Coats-Redfern method. The synthesized ligand, in comparison to their metal complexes also were screened for their antibacterial activity against bacterial species, Escherichia coli, Salmonella typhi, Bacillus subtillus, Staphylococcus aureus and Fungi (Aspergillus terreus and Aspergillus flavus). The activity data show that the metal complexes to be more potent/antimicrobial than the parent Shciff base ligand against one or more microbial species.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
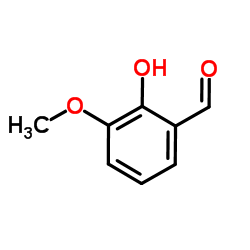 |
3-Methoxysalicylaldehyde
CAS:148-53-8 |
C8H8O3 |
|
3D-QSAR and molecular docking studies of benzaldehyde thiose...
2007-03-01 [Bioorg. Med. Chem. 15 , 2006-15, (2007)] |
|
A chemical screening approach reveals that indole fluorescen...
2009-09-01 [Bioorg. Med. Chem. Lett. 19 , 4952-7, (2009)] |
|
Design, synthesis and characterization of novel binary V(V)-...
2015-06-01 [J. Inorg. Biochem. 147 , 99-115, (2015)] |
|
Cu(II)-Dy(III) and Co(III)-Dy(III) based single molecule mag...
2015-08-07 [Dalton Trans. 44 , 13242-9, (2015)] |
|
Single-Molecule Magnetism, Enhanced Magnetocaloric Effect, a...
2015-10-26 [Chemistry 21 , 15639-50, (2015)] |