Quantum yields lower than unity in photo- induced dissociative electron transfers: the reductive cleavage of carbon tetrachloride.
L Pause, M Robert, J M Savéant
Index: ChemPhysChem 1(4) , 199-205, (2000)
Full Text: HTML
Abstract
It has been shown recently that the electrochemical reduction of carbon tetrachloride in N,N'-dimethylformamide follows a mechanism in which electron transfer and bond cleavage are concerted. We report here results concerning photoinduced electron transfer from the singlet excited state of two aromatic molecules, 2-ethyl-9,10-dimethoxyanthracene and perylene, to CCl4 , which is characterised by a quantum yield of complete quenching fragmentation ranging from 0.7 to 0.8. It is shown that a quantum yield below unity is compatible with a dissociative mechanism and arises from partitioning of the system at the intersection of the product- and ground-state potential energy surfaces. This phenomenon predominates over back electron transfer from the clustered fragments state. The photoinduced reductive cleavage of CCl4 thus provides a clear illustration of the recent theoretical prediction, that photoinduced dissociative electron transfers are not necessarily endowed with a unity quantum yield. This offers an opportunity to estimate the magnitude of the electronic matrix element that couples the fragmented product state and the ground reactant state.© 2000 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
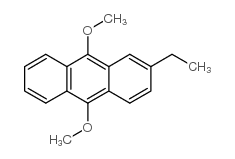 |
2-ETHYL-9,10-DIMETHOXYANTHRACENE
CAS:26708-04-3 |
C18H18O2 |
|
Application of a mathematical topological pattern of antihis...
2006-06-15 [J. Med. Chem. 49(12) , 3667-73, (2006)] |
|
Photocationic and radical polymerizations of epoxides and ac...
[J. Polym. Sci. A Polym. Chem. 41(23) , 3816-27, (2003)] |
|
Photosensitized reduction of sulfonium salts: Evidence for n...
[J. Am. Chem. Soc. 121(8) , 4364-68, (1999)] |
|
Novel N-methylbenzothiazolium salts as hardeners for epoxy a...
[J. Polym. Sci. A Polym. Chem. 41(23) , 3828-37, (2003)] |
|
Anthracene electron-transfer photosensitizers for onium salt...
[J. Photochem. Photobiol. A: Chem. 159(2) , 173-188, (2003)] |