New fluorescent substrates of microbial transglutaminase and its application to peptide tag-directed covalent protein labeling.
Noriho Kamiya, Hiroki Abe
Index: Methods Mol. Biol. 751 , 81-94, (2011)
Full Text: HTML
Abstract
Transglutaminase (TGase) is an enzyme that catalyzes the post-translational covalent cross-linking of Gln- and Lys-containing peptides and/or proteins according to its substrate specificity. We have recently designed a variety of Gln-donor fluorescent substrates of microbial transglutaminase (MTG) from Streptomyces mobaraensis and evaluated their potential use in MTG-mediated covalent protein labeling. The newly designed substrates are based on the relatively broad substrate recognition of MTG for the substitution of the N-terminal group of a conventional TGase substrate, benzyloxycarbonyl-L-glutaminylglycine (Z-QG). It is revealed that MTG is capable of accepting a diverse range of fluorophores in place of the N-terminal moiety of Z-QG when linked via a suitable linker. Here, we show the potential utility of a new fluorescent substrate for peptide tag-directed covalent protein labeling by employing fluorescein-4-isothiocyanate-β-Ala-QG as a model Gln-donor substrate for MTG.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
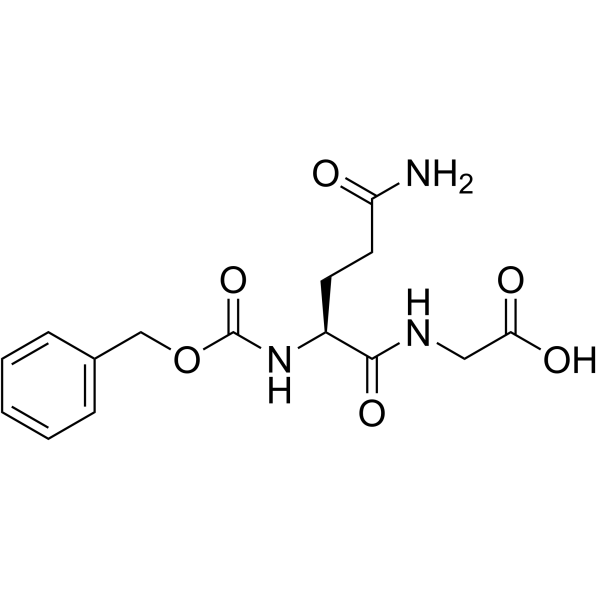 |
Z-Gln-Gly-OH
CAS:6610-42-0 |
C15H19N3O6 |
|
A direct continuous spectrophotometric assay for transglutam...
2000-10-01 [Anal. Biochem. 285 , 16-20, (2000)] |
|
Chemoenzymatic synthesis of neoglycopeptides: application to...
2001-05-04 [J. Org. Chem. 66 , 2948-56, (2001)] |
|
Kinetic analysis of the action of tissue transglutaminase on...
2003-08-12 [Biochemistry 42 , 9466-9481, (2003)] |
|
Transglutaminase-mediated synthesis of a DNA-(enzyme)n probe...
2011-05-02 [Chemistry 17 , 5387-5392, (2011)] |
|
Enzymatically catalyzed HES conjugation using microbial tran...
2009-11-01 [J. Pharm. Sci. 98 , 4420-4428, (2009)] |