Journal of Organic Chemistry
2001-05-04
Chemoenzymatic synthesis of neoglycopeptides: application to an alpha-Gal-terminated neoglycopeptide.
D Ramos, P Rollin, W Klaffke
Index: J. Org. Chem. 66 , 2948-56, (2001)
Full Text: HTML
Abstract
A novel methodology for the enzymatic preparation from suitably derivatized oligosaccharides of N-linked neoglycopeptides using the microbial glutaminyl-peptide gamma-glutamyl transferase, transglutaminase (TGase), is described. N-Allyl glycosides of various oligosaccharides were photochemically coupled with cysteamine to yield amino-terminated thioether spacers, which were accepted by transglutaminase to transamidate the side-chain gamma-carboxamide group in the dipeptide Z-Gln-Gly.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
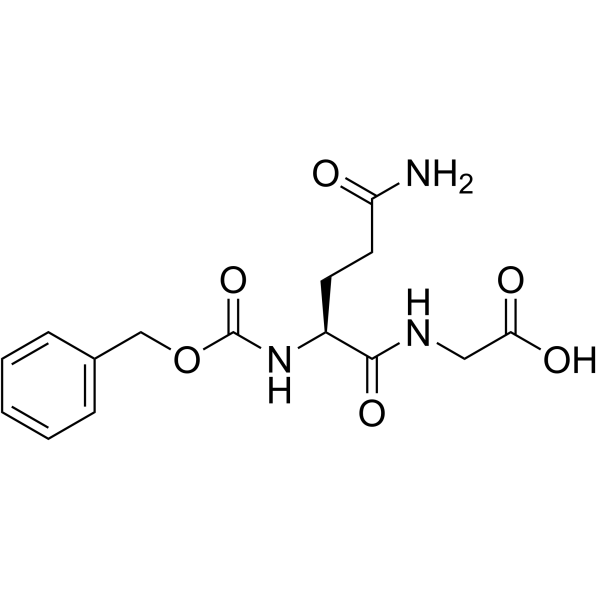 |
Z-Gln-Gly-OH
CAS:6610-42-0 |
C15H19N3O6 |
Related Articles:
More...
|
A direct continuous spectrophotometric assay for transglutam...
2000-10-01 [Anal. Biochem. 285 , 16-20, (2000)] |
|
Kinetic analysis of the action of tissue transglutaminase on...
2003-08-12 [Biochemistry 42 , 9466-9481, (2003)] |
|
Transglutaminase-mediated synthesis of a DNA-(enzyme)n probe...
2011-05-02 [Chemistry 17 , 5387-5392, (2011)] |
|
Enzymatically catalyzed HES conjugation using microbial tran...
2009-11-01 [J. Pharm. Sci. 98 , 4420-4428, (2009)] |
|
Functional glass surface displaying a glutamyl donor substra...
2010-05-01 [Biotechnol. J. 5 , 456-462, (2010)] |