Heterogeneous chemistry between PbSO4 and calcite microparticles using Raman microimaging.
Guillaume Falgayrac, Sobanska Sobanska, Jacky Laureyns, Claude Brémard
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 64(5) , 1095-101, (2006)
Full Text: HTML
Abstract
Currently, the smelting activities of lead and zinc are the loudest sources of local pollution by emission in the troposphere of dust of micrometer size containing PbSO(4). As the particles evolve in the troposphere, their chemical and physical properties - and hence their characteristics such as toxicity - change by accumulation of atmospheric heterogeneous reactions. Calcite (CaCO(3)) represents a large part of the mineral fraction in tropospheric aerosols with aerodynamic diameters less than 10 microm. The calcite particles are expected to react with PbSO(4) particles. In an effort to model the chemical behaviour of PbSO(4) individual particles in the troposphere, we present the in situ Raman imaging results during the course of the reactions in a water droplet of PbSO(4) particles with a calcite microcrystal surface. The computer-microcontrolled XY scanning and Z focusing of confocal Raman imaging combined with multivariate curve resolution (MCR) of Raman images have resolved the severe spectral overlaps of the Raman spectra which are not resolved by the spatial resolution of the instrument ( approximately 1 microm(3)). The results pointed out the identification and the mapping of Pb(3)(CO(3))(2)(OH)(2), PbCO(3) and CaSO(4).2H(2)O (gypsum) on the calcite surface.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
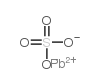 |
Lead sulfate
CAS:7446-14-2 |
O4PbS |
|
Chemical transformations of lead compounds under humid condi...
2013-02-01 [Environ. Geochem. Health 35(1) , 153-9, (2013)] |
|
Phosphate-induced lead immobilization from different lead mi...
2008-03-01 [Environ. Pollut. 152(1) , 184-92, (2008)] |
|
The effects of lead sulfate on new sealed lead acid batterie...
2000-04-01 [J. Emerg. Med. 18(3) , 305-9, (2000)] |
|
Mature experimental constructed wetlands treating urban wate...
2002-01-01 [Biotechnol. Prog. 18(6) , 1257-64, (2002)] |
|
Low temperature catalytic conversion of methane to methanol ...
2005-05-07 [Chem. Commun. (Camb.) (17) , 2238-40, (2005)] |