Study of pharmacokinetics and comparative bioavailability of nefopam 30 mg tablets in twelve fasting healthy Pakistani male young subjects: single-dose, randomized, two-period, two-treatment and two-way cross-over design.
Mahmood Ahmad, Muhammad Yaqoob, Ghulam Murtaza
Index: Med. Princ. Pract. 21(3) , 271-6, (2012)
Full Text: HTML
Abstract
To study the pharmacokinetics and comparative bioavailability of nefopam tablets (Acupan®).Experimentation of this study was based on a single-dose, two-sequence, cross-over randomized design using 12 fasting healthy Pakistani male young subjects. This validated LC/MS method was applied to a pharmacokinetic and bioavailability study in 12 fasting healthy Pakistani male subjects from the blood samples taken up to 24 h after an oral dose of one tablet of 30 mg nefopam in a double-blind, randomized, cross-over design.The mean maximum plasma concentration (C(max)) for the reference formulation was 60.71 ± 2.36 ng/ml (± SEM) and for test formulation 60.46 ± 1.30 ng/ml (± SEM). The mean time to reach maximum plasma concentration (T(max)) values of reference and test formulations was 1.63 ± 0.13 h (± SEM) and 1.83 ± 0.07 h (± SEM), respectively. The mean ± SEM values of AUC(0-)∞ for the reference and test formulations were 293.01 ± 16.09 ng·h/ml and 307.53 ± 8.99 ng·h/ml, respectively.The results showed that both formulations possessed almost the same relative bioavailability and pharmacokinetic parameters.Copyright © 2011 S. Karger AG, Basel.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
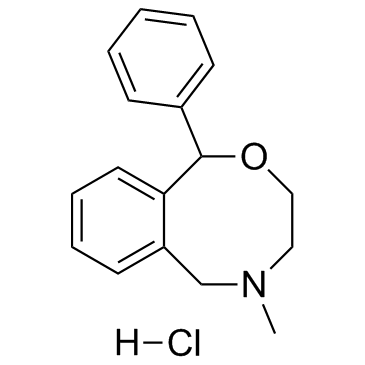 |
Nefopam hydrochloride
CAS:23327-57-3 |
C17H20ClNO |
|
Nefopam and meperidine are infra-additive on the shivering t...
2014-07-01 [Anesth. Analg. 119(1) , 58-63, (2014)] |
|
[Watch out for headaches at the end of a pregnancy! Do not m...
2010-04-01 [Gynecol. Obstet. Fertil. 38(4) , 278-82, (2010)] |
|
Median effective dose (ED50) of paracetamol and nefopam for ...
2013-03-01 [Minerva Anestesiol. 79(3) , 232-9, (2013)] |
|
Nefopam after total hip arthroplasty: role in multimodal ana...
2013-04-01 [Orthop. Traumatol. Surg. Res. 99(2) , 169-74, (2013)] |
|
Usefulness of nefopam in treating pain of severe uncomplicat...
2013-02-01 [Emerg. Med. J. 30(2) , 143-8, (2013)] |