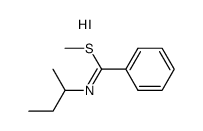
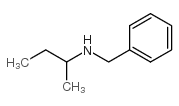
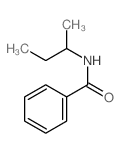
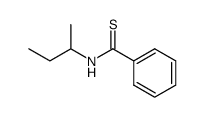
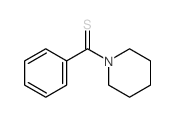
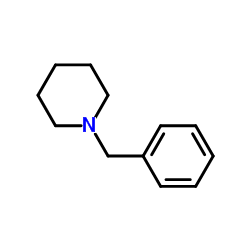
|
~52% |
|
~78% |
|
~% |
|
~44% |
|
~65% |
|
~49% |
|
~% |
|
~% |
|
~% |
|
~43% |
|
~% |
|
~% |
|
~65% |
|
~75% |
|
~% |
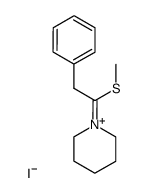

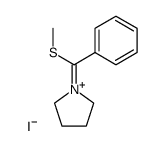
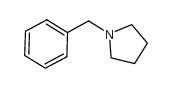
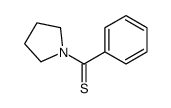
![N-Methyl-N-[α-Methylmercapto-benzyliden]-anilinium Structure](https://image.chemsrc.com/caspic/063/57513-33-4.png)
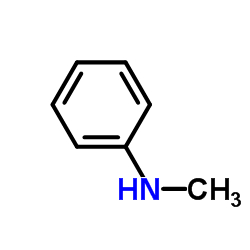

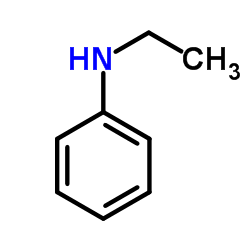

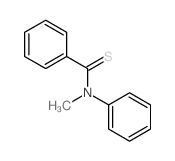
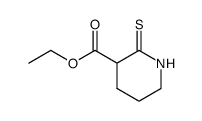
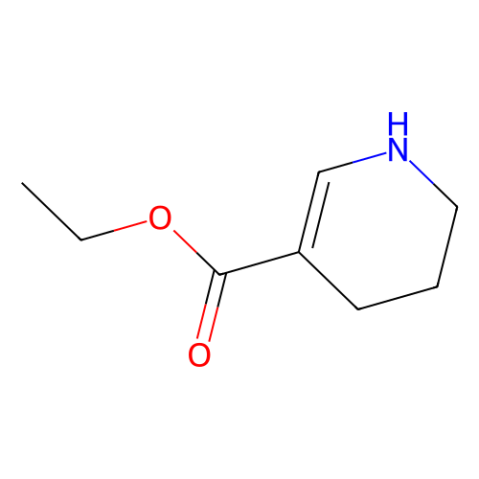
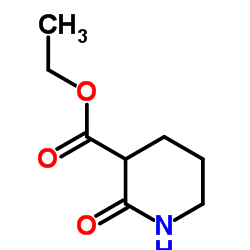

![1-[methylsulfanyl(phenyl)methylidene]piperidin-1-ium,iodide Structure](https://image.chemsrc.com/caspic/066/61135-82-8.png)