| Structure | Name/CAS No. | Articles |
|---|---|---|
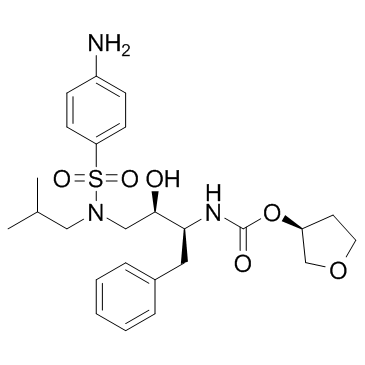 |
Amprenavir
CAS:161814-49-9 |
|
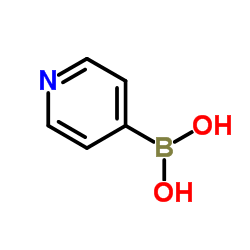 |
Pyridine-4-boronic acid
CAS:1692-15-5 |
|
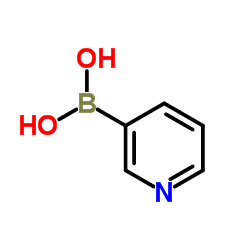 |
3-Pyridylboronic acid
CAS:1692-25-7 |