Double-blind study of cyamemazine and diazepam in the alcohol withdrawal syndrome.
Jean-Daniel Favre, Hervé Allain, Henri-Jean Aubin, Elisabeth Frija-Orvoen, Claudine Gillet, Michel Lejoyeux, Alain Payen, Michel Weber, Stéphanie Garcia-Acosta, Imane Kermadi, Michel Dib
Index: Hum. Psychopharmacol. Clin. Exp. 20(7) , 511-9, (2005)
Full Text: HTML
Abstract
Cyamemazine is an original phenothiazine derivative which showed similar efficacy and tolerability to lorazepam during ethanol withdrawal in mice. This study investigated cyamemazine for its efficacy and tolerability in alcohol-dependent patients electing an alcohol withdrawal procedure, in comparison with diazepam.A multicenter, randomized, double-blind study in 89 alcohol-dependent patients (CIWA-Ar score between 10 and 30), electing an alcohol withdrawal procedure, was used to find effective doses of cyamemazine and to compare it with diazepam for efficacy and tolerability. On day 1 (D(1)), cyamemazine or diazepam (50 mg and 10 mg capsule, respectively) were administered at hourly intervals to reduce CIWA-Ar = 5, up to a maximum of eight administrations. Starting from D(2), the compounds were given twice a day in progressively decreasing doses during a maximum period of 13 days (D(end)).At h(8) (8 h after the first treatment of D(1)), therapeutic success (CIWA-Ar score
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
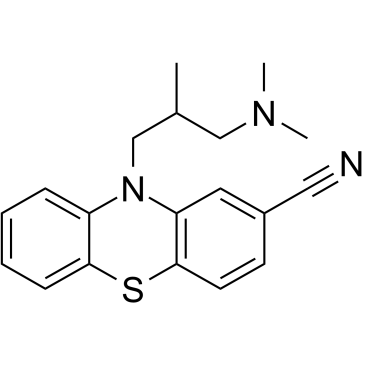 |
Cyamemazine
CAS:3546-03-0 |
C19H21N3S |
|
Identification and quantification of 35 psychotropic drugs a...
2015-03-01 [Int. J. Legal Med. 129(2) , 259-68, (2015)] |
|
Fatal intoxication with milnacipran
2008-01-01 [J. Forensic Leg. Med. 15(6) , 388-90, (2008)] |
|
Prise en charge médicamenteuse de l’anxiété chez le patient ...
2011-01-01 [Encephale 37 Suppl 1 , S83-9, (2011)] |
|
Characterization of human cytochrome P450 enzymes involved i...
2007-12-01 [Eur. J. Pharm. Sci. 32(4-5) , 357-66, (2007)] |
|
Potential role of cortical 5-HT(2A) receptors in the anxioly...
2012-07-30 [Psychiatry Res. 198(2) , 307-12, (2012)] |