Identification of intermediate and product from methemoglobin-catalyzed oxidation of o-phenylenediamine in two-phase aqueous-organic system.
Nitin Bhardwaj, Rajiv K Saxena
Index: Biochemistry. (Mosc.) 70(1) , 92-9, (2005)
Full Text: HTML
Abstract
Methemoglobin (metHb) was used as a mimetic enzyme for peroxidase to catalyze the oxidation reaction of o-phenylenediamine (OPDA) with H2O2 functioning as an oxidant. A reaction intermediate was obtained in two-phase aqueous-organic system and an absorption peak at 710 nm was confirmed to be that of the intermediate in relation to OPDA. The isolated product and intermediate were characterized by UV-Vis and IR spectrophotometry and HPLC-tandem mass spectrometry. The results showed that the product is 2,3-diaminophenazine, the molecular mass of the intermediate is 212 daltons, and a conceivable structure of the intermediate is suggested. Combining the catalyzed reaction mechanism of peroxidase and our experimental results, a conceivable oxidation reaction mechanism of OPDA and H2O2 using metHb as catalyst is proposed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
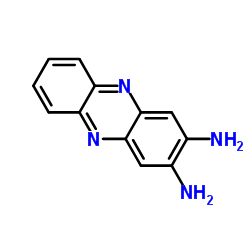 |
2,3-Diaminophenazine
CAS:655-86-7 |
C12H10N4 |
|
Spectrophotometric quantification of horseradish peroxidase ...
2010-12-15 [Anal. Biochem. 407(2) , 293-5, (2010)] |
|
Spectrophotometric quantification of lactose in solution wit...
2011-10-01 [Anal. Bioanal. Chem 401 , 2307-2310, (2011)] |
|
Photoinduced DNA cleavage and cellular damage in human derma...
2005-01-01 [Photochem. Photobiol. 81(1) , 89-95, (2005)] |
|
Experimental (FT-IR, FT-Raman and UV-Vis) spectra and theore...
2012-10-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 96 , 401-12, (2012)] |
|
Studies on the genotoxic effects of aminophenazines using tw...
1999-10-29 [Mutat. Res. 446(1) , 57-65, (1999)] |