| Structure | Name/CAS No. | Articles |
|---|---|---|
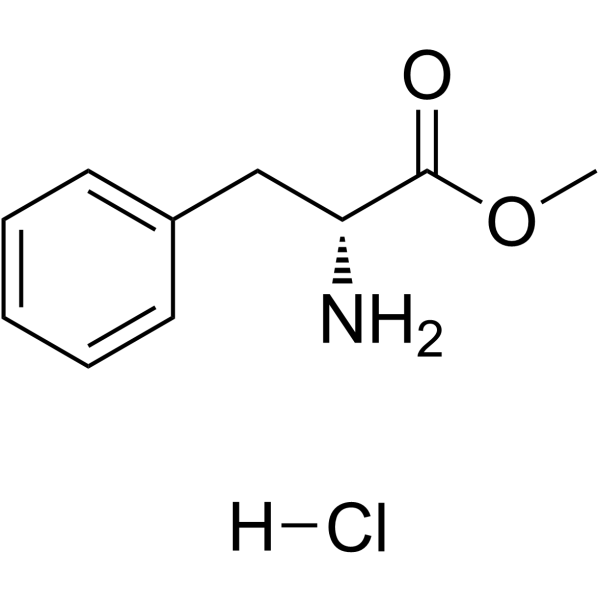 |
H-D-Phe-Ome HCl
CAS:13033-84-6 |
|
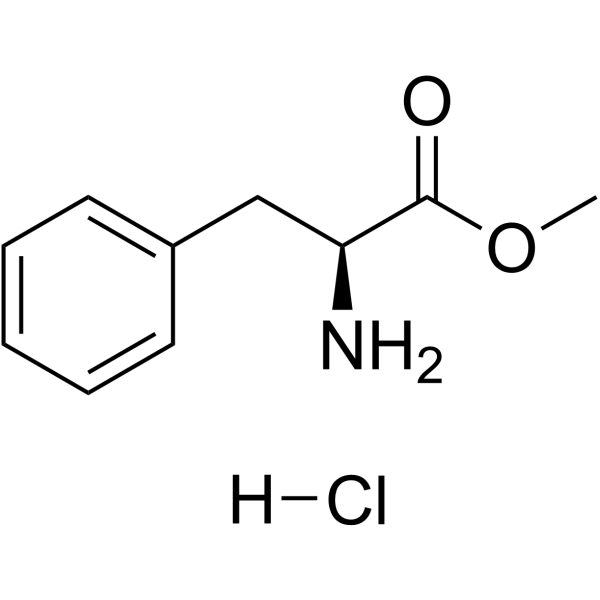 |
Methyl L-phenylalaninate hydrochloride
CAS:7524-50-7 |