Determination of WR-1065 in human blood by high-performance liquid chromatography following fluorescent derivatization by a maleimide reagent ThioGlo3.
Jun Chen, Zheng Lu, Theodore S Lawrence, David E Smith
Index: J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 819(1) , 161-7, (2005)
Full Text: HTML
Abstract
In order to improve the sensitivity and stability of human blood samples containing WR-1065 (i.e., active metabolite of the cytoprotective agent amifostine), a high-performance liquid chromatographic method was developed and validated using fluorescent derivatization with ThioGlo3. Using a sample volume of only 100 microl, the method was specific, sensitive (limit of quantitation=10 nM in deproteinized blood or 20 nM in whole blood), accurate (error < or = 3.2%) and reproducible (CV < or = 8.7%). In addition, the stability of WR-1065 in deproteinized and derivatized blood samples was assured for at least four weeks at -20 degrees C. This method should be particularly valuable in translating the kinetic-dynamic relationship of WR-1065 in preclinical models to that in cancer patients.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
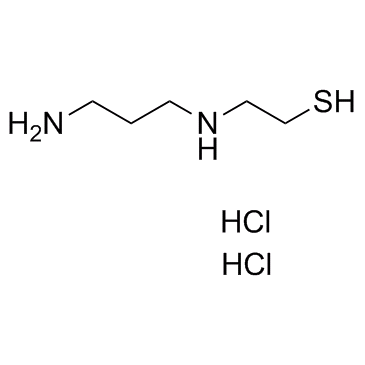 |
WR-1065
CAS:14653-77-1 |
C5H16Cl2N2S |
|
A manganese superoxide dismutase (SOD2)-mediated adaptive re...
2013-02-01 [Radiat. Res. 179(2) , 115-24, (2013)] |
|
Aminothiol WR1065 induces differential gene expression in th...
2005-06-02 [Oncogene 24(24) , 3964-75, (2005)] |
|
Clinical pharmacokinetics of amifostine and WR1065 in pediat...
2010-02-01 [Clin. Cancer Res. 16(3) , 1049-57, (2010)] |
|
WR1065 mitigates AZT-ddI-induced mutagenesis and inhibits vi...
2009-07-01 [Environ. Mol. Mutagen. 50(6) , 460-72, (2009)] |
|
Inhibition of human sperm respiration by 4-hydroperoxycyclop...
2009-01-01 [Fertil. Steril. 91(1) , 173-8, (2009)] |