Involvement of common intermediate 3-hydroxy-L-kynurenine in chromophore biosynthesis of quinomycin family antibiotics.
Yuki Hirose, Kenji Watanabe, Atsushi Minami, Takemichi Nakamura, Hiroki Oguri, Hideaki Oikawa
Index: J. Antibiot. 64(1) , 117-22, (2011)
Full Text: HTML
Abstract
Quinomycin antibiotics, represented by echinomycin, are an important class of antitumor antibiotics. We have recently succeeded in identification of biosynthetic gene clusters of echinomycin and SW-163D, and have achieved heterologous production of echinomycin in Escherichia coli. In addition, we have engineered echinomycin non-ribosomal peptide synthetase to generate echinomycin derivatives. However, the biosynthetic pathways of intercalative chromophores quinoxaline-2-carboxylic acid (QXC) and 3-hydroxyquinaldic acid (HQA), which are important for biological activity, were not fully elucidated. Here, we report experiments involving incorporation of a putative advanced precursor, (2S, 3R)-[6'-(2)H]-3-hydroxy-L-kynurenine, and functional analysis of the enzymes Swb1 and Swb2 responsible for late-stage biosynthesis of HQA. On the basis of these experimental results, we propose biosynthetic pathways for both QXC and HQA through the common intermediate 3-hydroxy-L-kynurenine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
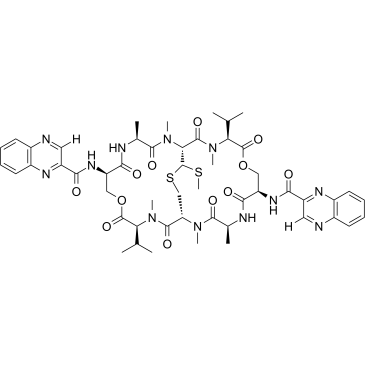 |
Echinomycin
CAS:512-64-1 |
C51H64N12O12S2 |
|
Regulatory effect of hypoxia-inducible factor-1α on hCG-stim...
2012-01-01 [J. Reprod. Dev. 58(6) , 678-84, (2012)] |
|
Interaction of an echinomycin-DNA complex with manganese ion...
2009-07-01 [Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65(Pt 7) , 660-4, (2009)] |
|
Effects of echinomycin on endothelin-2 expression and ovulat...
2012-10-31 [Exp. Mol. Med. 44(10) , 615-21, (2012)] |
|
A novel role for the transcription factor HIF-1α in the form...
2012-08-15 [Biochem. J. 446(1) , 159-63, (2012)] |
|
Echinomycin decreases induction of vascular endothelial grow...
2012-04-01 [Basic Clin Pharmacol Toxicol. 110(4) , 327-34, (2012)] |