| Structure | Name/CAS No. | Articles |
|---|---|---|
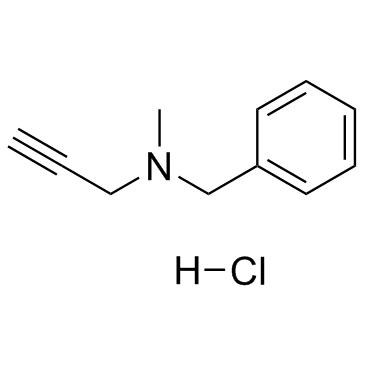 |
pargyline hydrochloride
CAS:306-07-0 |
|
 |
Cyclopentenone
CAS:930-30-3 |