Simultaneous determination of a new gastrointestinal prokinetic agent (HSR-803) and its metabolites in human serum and urine by high-performance liquid chromatography using automated column-switching.
E Takahara, H Fukuoka, T Takagi, O Nagata, H Kato
Index: J. Chromatogr. A. 576(1) , 174-8, (1992)
Full Text: HTML
Abstract
A method based on high-performance liquid chromatography using column-switching is described for the simultaneous determination of HSR-803 and its metabolites in human serum and urine. The system uses a six-port valve with a Nucleosil CN pre-column for on-line sample clean-up, and direct injection of samples. The limits of quantitation in serum and urine were 5 and 20 ng/ml for HSR-803 and 50 and 200 ng/ml for the metabolites, respectively. The coefficients of variation for the intra- and inter-day accuracies were between 0.8 and 7.1% for each compound. This method was applied to the pharmacokinetic studies in humans after oral administration of HSR-803.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
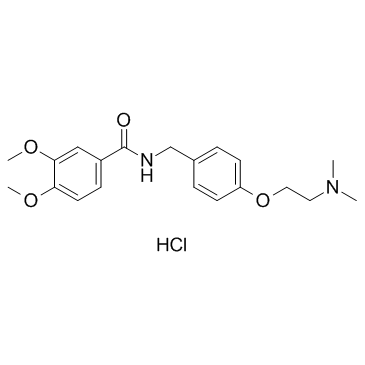 |
Itopride hydrochloride
CAS:122892-31-3 |
C20H27ClN2O4 |
|
Effect of reversible ligands on oxime-induced reactivation o...
2015-02-03 [Toxicol. Lett. 232(3) , 557-65, (2015)] |
|
QbD-enabled systematic development of gastroretentive multip...
2016-01-01 [Drug Deliv. 23 , 437-51, (2016)] |
|
[Effect of HSR-803 on gastrointestinal motility].
1989-12-01 [Nihon Heikatsukin Gakkai Zasshi 25(6) , 313-5, (1989)] |
|
A novel water-soluble dopamine-2 antagonist with anticholine...
1990-08-01 [Gastroenterology 99(2) , 401-8, (1990)] |
|
Stimulatory effect of N-[4-[2-(dimethylamino)-ethoxy] benzyl...
1991-07-01 [Jpn. J. Pharmacol. 56(3) , 261-9, (1991)] |