Antimicrobial lipopeptaibol trichogin GA IV: role of the three Aib residues on conformation and bioactivity.
Marta De Zotti, Barbara Biondi, Yoonkyung Park, Kyung-Soo Hahm, Marco Crisma, Claudio Toniolo, Fernando Formaggio
Index: Amino Acids 43(4) , 1761-77, (2012)
Full Text: HTML
Abstract
The lipopeptaibol trichogin GA IV is a natural, non-ribosomally synthesized, antimicrobial peptide remarkably resistant to the action of hydrolytic enzymes. This feature may be connected to the multiple presence in its sequence of the non-coded residue α-aminoisobutyric acid (Aib), which is known to be responsible for the adoption of particularly stable helical structures already at the level of short peptides. To investigate the role of Aib residues on the 3D-structure and bioactivity of trichogin GA IV, we synthesized and fully characterized four analogs where one or two Aib residues are replaced by L-Leu. Our extensive conformational studies (including an X-ray diffraction analysis) and biological assays performed on these analogs showed that the Aib to L-Leu replacements do not affect the resistance to proteolysis, but modulate the bioactivity of trichogin GA IV in a 3D-structure related manner.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
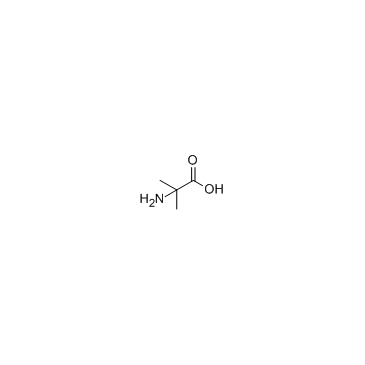 |
2-Aminoisobutyric acid
CAS:62-57-7 |
C4H9NO2 |
|
Metabolomic profiles delineate potential role for sarcosine ...
2009-02-12 [Nature 457(7231) , 910-4, (2009)] |
|
Ion-pairing reversed-phase liquid chromatography fractionati...
2011-01-01 [J. Chromatogr. A. 1218(23) , 3689-94, (2011)] |
|
Isotope-labeled differential profiling of metabolites using ...
2015-09-30 [Rapid Commun. Mass Spectrom. 29 , 1632-40, (2015)] |
|
Three-dimensional quantitative structure-activity relationsh...
2011-11-01 [Bioorg. Med. Chem. 19 , 6409-18, (2011)] |
|
Specificity of the volume-activated amino acid efflux pathwa...
2011-03-01 [Gen. Physiol. Biophys. 30(1) , 45-51, (2011)] |