Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A synthase by antibiotic 1233A and other beta-lactones.
R J Mayer, P Louis-Flamberg, J D Elliott, M Fisher, J Leber
Index: Biochem. Biophys. Res. Commun. 169(2) , 610-6, (1990)
Full Text: HTML
Abstract
3-Hydroxy-3-methylglutaryl CoA synthase was shown to be inhibited in a time-dependent, irreversible manner by compounds containing the substituted beta-lactone functionality found in the natural product 1233A. The rate of inactivation (kinact) was found to approach the rate of catalysis (kcat). The inactivation was irreversible over several hours. A related compound lacking the hydroxymethyl substituent on the beta-lactone ring is a reversible inhibitor and is competitive with respect to acetylCoA. The results are consistent with beta-lactone ring opening by the active site Cys to form an enzyme bound thioester.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
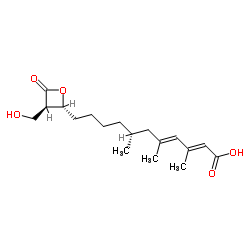 |
hymeglusin
CAS:29066-42-0 |
C18H28O5 |
|
Characterization of peroxisomal 3-hydroxy-3-methylglutaryl c...
2000-01-11 [Biochemistry 39(1) , 237-47, (2000)] |
|
Binding site for fungal beta-lactone hymeglusin on cytosolic...
2004-02-27 [Biochim. Biophys. Acta 1636(1) , 22-8, (2004)] |
|
Biosynthesis of antibiotic 1233A (F-244) and preparation of ...
1992-04-01 [J. Antibiot. 45(4) , 563-7, (1992)] |
|
Isopentenoid synthesis in eukaryotic cells. An initiating ro...
1993-04-01 [Arch. Biochem. Biophys. 302(1) , 265-71, (1993)] |
|
Inhibition of hepatic cholesterol biosynthesis by a 3-hydrox...
1993-01-01 [Life Sci. 52(19) , 1595-600, (1993)] |