Biosynthesis of antibiotic 1233A (F-244) and preparation of [14C]1233A.
H Kumagai, H Tomoda, S Omura
Index: J. Antibiot. 45(4) , 563-7, (1992)
Full Text: HTML
Abstract
The biosynthesis of antibiotic 1233A (F-244) was studied by feeding 13C-labeled precursors to the producing organism, Scopulariopsis sp. F-244. 13C NMR spectroscopy established that 1233A is derived from 4 methionines and 7 acetates. Seven acetates are condensed to form a hexaketide and 4 methyl residues from methionine are introduced into the main skeleton. The beta-lactone is derived from the alpha-carboxylic acid of the hexaketide. Since methionine was efficiently incorporated into 1233A, radiolabeled 1233A was prepared biosynthetically by feeding [14C]methionine to the producer. As a result, [14C]1233A was obtained with high specific radioactivity (27.2 muCi/mumols).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
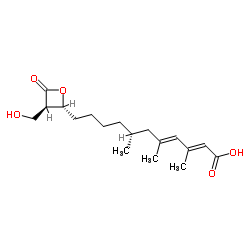 |
hymeglusin
CAS:29066-42-0 |
C18H28O5 |
|
Characterization of peroxisomal 3-hydroxy-3-methylglutaryl c...
2000-01-11 [Biochemistry 39(1) , 237-47, (2000)] |
|
Binding site for fungal beta-lactone hymeglusin on cytosolic...
2004-02-27 [Biochim. Biophys. Acta 1636(1) , 22-8, (2004)] |
|
Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A synthase...
1990-06-15 [Biochem. Biophys. Res. Commun. 169(2) , 610-6, (1990)] |
|
Isopentenoid synthesis in eukaryotic cells. An initiating ro...
1993-04-01 [Arch. Biochem. Biophys. 302(1) , 265-71, (1993)] |
|
Inhibition of hepatic cholesterol biosynthesis by a 3-hydrox...
1993-01-01 [Life Sci. 52(19) , 1595-600, (1993)] |