Pharmacokinetics of temozolomide administered in combination with O6-benzylguanine in children and adolescents with refractory solid tumors.
Holly J Meany, Katherine E Warren, Elizabeth Fox, Diane E Cole, Alberta A Aikin, Frank M Balis
Index: Cancer Chemother. Pharmacol. 65(1) , 137-42, (2009)
Full Text: HTML
Abstract
Temozolomide pharmacokinetics were evaluated in children receiving concurrent O(6)-benzylguanine (O(6)BG), which enhanced the hematological toxicity of temozolomide.Temozolomide was administered orally, daily for 5 days starting at 28 mg/m(2) per day with escalations to 40, 55, 75 and 100 mg/m(2) per day with O(6)BG intravenously daily for 5 days at doses of 60, 90 or 120 mg/m(2) per day. Plasma samples were drawn over 48 h after the day 5 dose. Temozolomide was quantified with a validated HPLC/tandem mass spectroscopic assay.Temozolomide was rapidly absorbed (mean T (max), 2.1 h). The mean apparent clearance (CL/F) (96 mL/min/m(2)) was similar to the CL/F for temozolomide alone and was not age- or gender-dependent. There was minimal inter-patient variability.The enhanced hematologic toxicity resulting from combining O(6)BG with temozolomide does not appear to be the result of a pharmacokinetic interaction between the agents.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
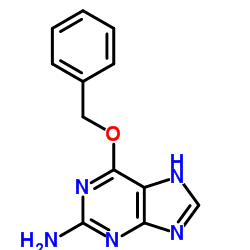 |
O6-Benzylguanine
CAS:19916-73-5 |
C12H11N5O |
|
Differential expression of miR200a-3p and miR21 in grade II-...
2014-07-01 [Cancer Biol. Ther. 15(7) , 938-50, (2014)] |
|
Formation and repair of pyridyloxobutyl DNA adducts and thei...
2012-10-15 [Chem. Res. Toxicol. 25(10) , 2167-78, (2012)] |
|
Evaluation of novel imidazotetrazine analogues designed to o...
2015-01-01 [Mol. Cancer Ther. 14(1) , 111-9, (2015)] |
|
4-nitrobenzyloxycarbonyl derivatives of O(6)-benzylguanine a...
2011-11-10 [J. Med. Chem. 54(21) , 7720-8, (2011)] |
|
Phase I clinical trial of O6-benzylguanine and topical carmu...
2012-05-01 [Arch. Dermatol. 148(5) , 613-20, (2012)] |