Phase I clinical trial of O6-benzylguanine and topical carmustine in the treatment of cutaneous T-cell lymphoma, mycosis fungoides type.
Narin Apisarnthanarax, Gary S Wood, Seth R Stevens, Sean Carlson, Derek V Chan, Lili Liu, Sarolta K Szabo, Pingfu Fu, Anita C Gilliam, Stanton L Gerson, Scot C Remick, Kevin D Cooper
Index: Arch. Dermatol. 148(5) , 613-20, (2012)
Full Text: HTML
Abstract
To evaluate the toxic effects and maximum tolerated dose of topical carmustine [1,3-bis(2-chloroethyl)-1-nitrosourea] following intravenous O6-benzylguanine in the treatment of cutaneous T-cell lymphoma (CTCL), and to determine pharmacodynamics of O6-alkylguanine DNA alkyltransferase activity in treated CTCL lesions.Open-label, dose-escalation, phase I trial.Dermatology outpatient clinic and clinical research unit at a university teaching hospital.A total of 21 adult patients (11 male, 10 female)with early-stage (IA-IIA) refractory CTCL, mycosis fungoides type, treated with topical carmustine following intravenous O6-benzylguanine.Treatment once every 2 weeks with 120 mg/m(2) intravenous O6-benzylguanine followed 1 hour later by whole-body, low-dose topical carmustine starting at 10 mg, with 10-mg incremental dose-escalation in 3 patient cohorts. Cutaneous T-cell lymphoma lesional skin biopsy specimens were taken at baseline and 6 hours, 24 hours, and 1 week after the first O6-benzylguanine infusion for analysis of O6-alkylguanine-DNA alkyltransferase activity.Clinical response measured by physical examination and severity-weighted assessment tool measurements, safety data acquired by review of adverse events at study visits, and O6-alkylguanine-DNA alkyltransferase activity in treated lesion skin biopsy specimens.A minimal toxic effect was observed through the 40-mg carmustine dose level with 76% of adverse events being grade 1 based on the National Cancer Institute Common Terminology Criteria for Adverse Events. Mean baseline O6-alkylguanine-DNA alkyltransferase activity in CTCL lesions was 3 times greater than in normal controls and was diminished by a median of 100% at 6 and 24 hours following O6-benzylguanine with recovery at 1 week. Clinical disease reduction correlated positively with O6-alkylguanine-DNA alkyltransferase activity at 168 hours (P=.02) and inversely with area under the curve of O6-alkylguanine-DNA alkyltransferase over 1 week (P=.01). Twelve partial responses and 4 complete responses were observed (overall response, 76% [95% CI, 0.55-0.89]). Five patients discontinued therapy owing to adverse events with a possible, probable, or definite relationship to the study drug.O6-benzylguanine significantly depletes O6-alkylguanine-DNA alkyltransferase in CTCL lesions and in combination with topical carmustine is well tolerated and shows meaningful clinical responses in CTCL at markedly reduced total carmustine treatment doses.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
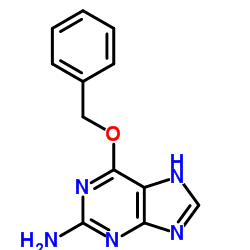 |
O6-Benzylguanine
CAS:19916-73-5 |
C12H11N5O |
|
Differential expression of miR200a-3p and miR21 in grade II-...
2014-07-01 [Cancer Biol. Ther. 15(7) , 938-50, (2014)] |
|
Formation and repair of pyridyloxobutyl DNA adducts and thei...
2012-10-15 [Chem. Res. Toxicol. 25(10) , 2167-78, (2012)] |
|
Evaluation of novel imidazotetrazine analogues designed to o...
2015-01-01 [Mol. Cancer Ther. 14(1) , 111-9, (2015)] |
|
4-nitrobenzyloxycarbonyl derivatives of O(6)-benzylguanine a...
2011-11-10 [J. Med. Chem. 54(21) , 7720-8, (2011)] |
|
MGMT inhibition restores ERα functional sensitivity to antie...
2012-01-01 [Mol. Med. 18 , 913-29, (2012)] |