| Structure | Name/CAS No. | Articles |
|---|---|---|
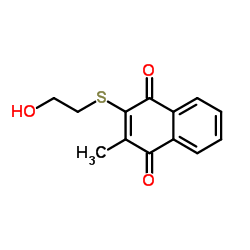 |
Inulinase fromAspergillus niger
CAS:9025-67-6 |
|
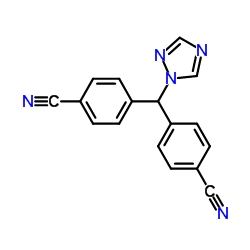 |
Inulin
CAS:9005-80-5 |