Inhibition of adenylyl cyclase by acyclic nucleoside phosphonate antiviral agents.
I Shoshani, W H Laux, C Périgaud, G Gosselin, R A Johnson
Index: J. Biol. Chem. 274(49) , 34742-4, (1999)
Full Text: HTML
Abstract
Acyclic derivatives of adenine, known as highly effective nucleotide analogs with broad spectrum antiviral activity, were evaluated for potential cross-reactivity with adenylyl cyclases, a family of membrane-bound enzymes that share putative topologies at their catalytic sites with oligonucleotide polymerases and reverse transcriptases. A series of derivatives of 9-(2-phosphonylmethoxyethyl)adenine (PMEA) inhibited a preparation of adenylyl cyclase derived from rat brain with IC(50) values that ranged from 66 microM (PMEA) to 175 nM for its diphosphate derivative (PMEApp) and mimics of it. PMEApp mimics included PMEAp(NH)p, PMEAp(CH(2))p, PMEAp(CX(2))p (X = fluorine, chlorine, or bromine), PMEAp(CHX)pp, and PMEAp(C(OH)CH(3)pp. The data suggest that inhibition of adenylyl cyclases may contribute to the therapeutic action of some of these or similar compounds or constitute part of their side effects in therapeutic settings.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
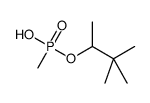 |
pinacolyl methylphosphonate
CAS:616-52-4 |
C7H17O3P |
|
On-line solid phase extraction-liquid chromatography-mass sp...
2013-01-25 [Anal. Chim. Acta 761 , 109-16, (2013)] |
|
Capillary ion electrophoresis screening of nerve agent degra...
1998-10-16 [J. Chromatogr. A. 824(1) , 125-34, (1998)] |
|
On-line solid-phase extraction liquid chromatography-continu...
1999-02-19 [J. Chromatogr. A. 833(2) , 169-79, (1999)] |
|
Derivatization of organophosphorus nerve agent degradation p...
2007-06-01 [Anal. Bioanal. Chem 388(4) , 809-23, (2007)] |
|
Detection of degradation products of chemical warfare agents...
2008-05-01 [Analyst 133(5) , 588-95, (2008)] |