Development of a two-step route to 3-PBC and βCCt, two agents active against alcohol self-administration in rodent and primate models.
Ojas A Namjoshi, Angelica Gryboski, German O Fonseca, Michael L Van Linn, Zhi-jian Wang, Jeffrey R Deschamps, James M Cook
Index: J. Org. Chem. 76(11) , 4721-7, (2011)
Full Text: HTML
Abstract
To gain access to 3-propoxy-β-carboline hydrochloride (3-PBC·HCl) (1·HCl) and β-carboline-3-carboxylate-tert-butyl ester (βCCt) (2), potential clinical agents active against alcohol self-administration, a two-step route was developed. This process involves a palladium-catalyzed Buchwald-Hartwig coupling and an intramolecular Heck reaction. This two-step route provides rapid access to multigram quantities of 3-PBC (1) and βCCt (2), as well as analogues for studies of alcohol self-administration. The overall yield of 3-PBC (1) was improved from 8% to 50% by this route.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
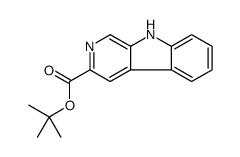 |
tert-butyl beta-carboline-3-carboxylate
CAS:93835-05-3 |
C16H16N2O2 |
|
βCCT, an antagonist selective for α(1)GABA(A) receptors, rev...
2013-02-01 [Brain Res. Bull. 91 , 1-7, (2013)] |
|
Apparent pA2 values of benzodiazepine antagonists and partia...
1999-09-01 [J. Pharmacol. Exp. Ther. 290(3) , 1222-9, (1999)] |
|
Cognition-impairing effects of benzodiazepine-type drugs: ro...
2013-03-01 [Pharmacol. Biochem. Behav. 104 , 62-8, (2013)] |
|
Bidirectional effects of benzodiazepine binding site ligands...
2005-07-01 [Psychopharmacology 180(3) , 455-65, (2005)] |
|
The role of α1 and α5 subunit-containing GABAA receptors in ...
2012-04-01 [Behav. Pharmacol. 23(2) , 191-7, (2012)] |