Synthesis of [19, 35, 36-(13)C(3)]-labeled TAK779 as a molecular probe.
Hiroyuki Konno, Saburo Aimoto, Steven O Smith, Kazuto Nosaka, Kenichi Akaji
Index: Bioorg. Med. Chem. 17(16) , 5769-74, (2009)
Full Text: HTML
Abstract
N,N-Dimethyl-N-[4-[[[2-(4-methylphenyl)-6,7-dihydro-5H-benzocyclohepten-8-yl]carbonyl]amino]benzyl]tetrahydro-2H-pyran-4-aminium chloride (TAK779) is a potent and selective non-peptide CCR5 antagonist. To use a site-specifically labeled form as a molecular probe, TAK779 containing (13)C at positions C19, 35, and 36 was produced. A commercially available [(13)C]-methyl iodide was employed for the labeling. Starting from a known carboxylic acid segment containing no labeled carbon, the labeled TAK779 was constructed by the successive coupling of [(13)C]-labeled tolyl boronic ester by the Suzuki-Miyaura reaction and a [(13)C]-labeled aniline segment by amide bond formation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
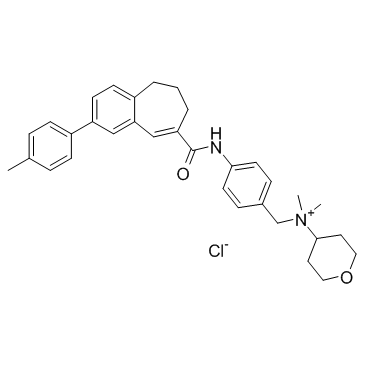 |
TAK-779
CAS:229005-80-5 |
C33H39ClN2O2 |
|
Effects of a calcineurin inhibitor, FK506, and a CCR5/CXCR3 ...
2011-07-01 [Transpl. Immunol. 25(1) , 49-55, (2011)] |
|
Evolution of CCR5 antagonist resistance in an HIV-1 subtype ...
2010-12-01 [J. Acquir. Immune Defic. Syndr. 55(4) , 420-7, (2010)] |
|
HIV-1 clinical isolates resistant to CCR5 antagonists exhibi...
2012-01-01 [J. Virol. 86(2) , 1119-28, (2012)] |
|
C-C chemokine receptor type 5 (CCR5) utilization of transmit...
2010-12-01 [J. Gen. Virol. 91(Pt 12) , 2965-73, (2010)] |
|
Blockade of Th1 chemokine receptors ameliorates pulmonary gr...
2011-08-01 [Eur. Respir. J. 38(2) , 415-24, (2011)] |