| Structure | Name/CAS No. | Articles |
|---|---|---|
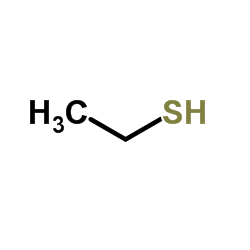 |
Ethanethiol
CAS:75-08-1 |
|
 |
Sodium ethanethiolate
CAS:811-51-8 |
|
 |
SODIUM 1-PROPANETHIOLATE
CAS:6898-84-6 |
|
 |
1-Propanethiol
CAS:107-03-9 |