| Structure | Name/CAS No. | Articles |
|---|---|---|
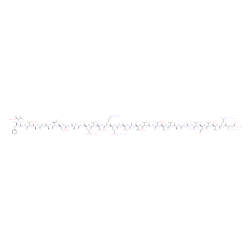 |
α-Synuclein (61-95) (human) trifluoroacetate salt
CAS:154040-19-4 |
|
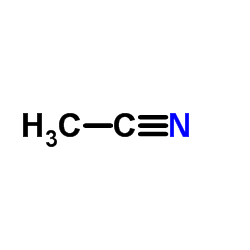 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Acetylcysteine(N-acetylcysteine)
CAS:616-91-1 |
|
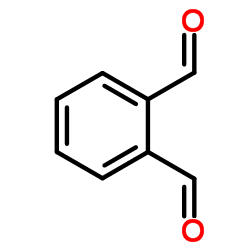 |
o-Phthalaldehyde
CAS:643-79-8 |
|
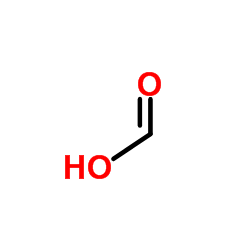 |
Formic Acid
CAS:64-18-6 |
|
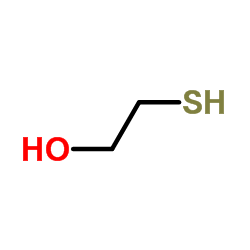 |
mercaptoethanol
CAS:60-24-2 |
|
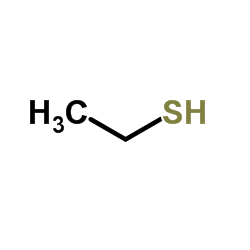 |
Ethanethiol
CAS:75-08-1 |
|
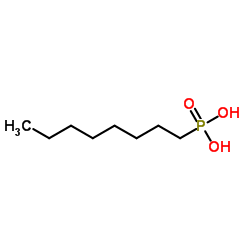 |
1-Octylphosphonic acid
CAS:4724-48-5 |