Tolerability and pharmacokinetics of two formulations of megestrol acetate under fed conditions in healthy volunteers.
Virginia Fuochi, Giulio Petronio Petronio, Edmondo Lissandrello, Pio Maria Furneri
Index: Clin. Ther. 37(2) , 439-47, (2015)
Full Text: HTML
Abstract
Megestrol acetate oral suspension is an appetite stimulant indicated for cachexia. It is available in a conventional formulation and as a nanocrystal dispersion. The aim of this study was to compare the tolerability and pharmacokinetics of these formulations under fed conditions in healthy Korean volunteers.This was a randomized, single-dose, 3-treatment, 3-period, 6-sequence, crossover study in healthy Korean volunteers. In each period, participants received single oral doses of conventional formulation 800 mg/20 mL (reference), nanocrystal dispersion 650 mg/5.2 mL (test 1), and nanocrystal dispersion 675 mg/5.4 mL (test 2) after a high-calorie, high-fat meal. The periods were separated by a washout period of 14 days. Serial blood samples were collected up to 120 hours after dosing. The plasma concentrations of megestrol acetate were determined with a validated LC-MS/MS method. Pharmacokinetic parameters were obtained by noncompartmental analysis. Tolerability was assessed by physical examinations, vital signs, clinical laboratory test results, and electrocardiograms.Thirty-eight healthy volunteers completed the study. The geometric mean ratios of the AUC(last) and C(max) for test 1/reference were 0.88 (90% CI, 0.84-0.92) and 1.07 (90% CI, 0.99-1.15), respectively. The geometric mean ratios of the AUC(last) and C(max) for test 2/reference were 0.88 (90% CI, 0.84-0.93) and1.03 (90% CI, 0.96-1.10), respectively. All formulations were well tolerated.The pharmacokinetic characteristics and tolerability of the 2 megestrol acetate formulations are similar in fed volunteers and suggest no relevant difference in tolerability. ClinicalTrials.gov identifier: NCT01342055.Copyright © 2015 Elsevier HS Journals, Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
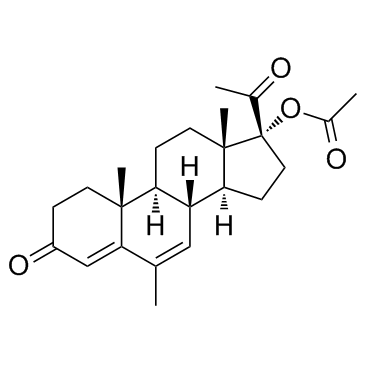 |
Megestrol acetate
CAS:595-33-5 |
C24H32O4 |
|
Cheminformatics analysis of assertions mined from literature...
2010-01-01 [Chem. Res. Toxicol. 23 , 171-83, (2010)] |
|
Translating clinical findings into knowledge in drug safety ...
2011-12-01 [J. Sci. Ind. Res. 65(10) , 808, (2006)] |
|
Multi-target spectral moment QSAR versus ANN for antiparasit...
2010-03-15 [Bioorg. Med. Chem. 18 , 2225-31, (2010)] |
|
Quantitative structure-activity relationship and complex net...
2008-11-13 [J. Med. Chem. 51 , 6740-51, (2008)] |
|
Fenretinide: a novel treatment for endometrial cancer.
2014-01-01 [PLoS ONE 9(10) , e110410, (2014)] |