Pharmacokinetics of oral pramiracetam in normal volunteers.
T Chang, R M Young, J R Goulet, G J Yakatan
Index: J. Clin. Pharmacol. 25(4) , 291-5, (1985)
Full Text: HTML
Abstract
The pharmacokinetics of pramiracetam, a new, investigational, cognition activator, were assessed in normal male volunteers as part of a clinical tolerance study. In a double-blind, randomized design, two groups of six subjects each received alternating placebo and single 400, 800, 1,200, and 1,600 mg oral doses of pramiracetam after an overnight fast. Mean (+/- SD) peak plasma concentrations of the four dose groups (2.71 +/- 0.54, 5.40 +/- 1.34, 6.13 +/- 0.71, 8.98 +/- 0.71 micrograms/mL) were attained between two to three hours following drug administration. The harmonic mean elimination half-life (4.5-6.5 hours), the mean total body clearance (4.45-4.85 mL/min/kg), the mean renal clearance (1.83-3.00 mL/min/kg), and the mean apparent volume of distribution (1.82-2.94 L/kg) were independent of dose, whereas the peak plasma concentrations and area under the curves increased as a linear function of dose. No significant side effects were observed at any dose level.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
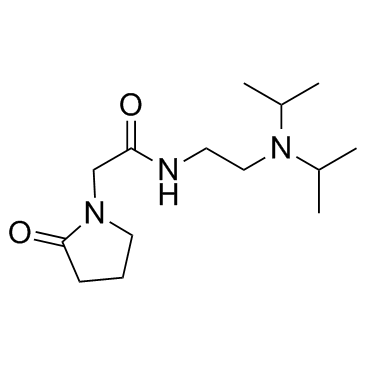 |
pramiracetam
CAS:68497-62-1 |
C14H27N3O2 |
|
The effects of cholinergic drugs support an avoidance learni...
1986-10-01 [Neuropharmacology 25(10) , 1161-6, (1986)] |
|
The memory-enhancing effects of the piracetam-like nootropic...
1989-05-01 [Behav. Brain Res. 33(1) , 79-82, (1989)] |
|
Gas chromatographic assay of pramiracetam in human plasma us...
[J. Chromatogr. A. 274 , 346-9, (1983)] |
|
Pharmacokinetics of pramiracetam in healthy volunteers after...
1992-01-01 [Int. J. Clin. Pharmacol. Res. 12(3) , 129-32, (1992)] |
|
Pramiracetam and epileptic after-discharges in young rats af...
1989-04-01 [Act. Nerv. Super. (Praha.) 31(1) , 68-9, (1989)] |