Pharmacokinetics of pramiracetam in healthy volunteers after oral administration.
A Auteri, P Blardi, G Celasco, G Segre, R Urso
Index: Int. J. Clin. Pharmacol. Res. 12(3) , 129-32, (1992)
Full Text: HTML
Abstract
The pharmacokinetics of pramiracetam was assessed using an HPLC method after oral administration of two different formulations of 600 mg (a solution and a tablet) of pramiracetam to 11 fasting volunteers. The mean kinetic parameters were: t1 = 4.7 +/- 2.4 - 4.3 +/- 2.2 h, AUC = 57.6 +/- 43.6 - 47.2 +/- 33.9 micrograms h/ml, Cmax = 6.80 +/- 3.2 - 5.80 +/- 3.3 micrograms/ml for the solution and the tablet respectively. The plasma profile of pramiracetam proved to be not highly affected by the formulation, only that the absorption rate was faster after oral administration of the drug in solution than after administration as a tablet. The half-life was very variable between subjects [2-8 hours], but less variable within subjects and it was unaffected by the formulation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
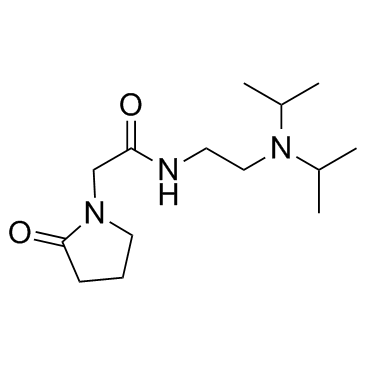 |
pramiracetam
CAS:68497-62-1 |
C14H27N3O2 |
|
The effects of cholinergic drugs support an avoidance learni...
1986-10-01 [Neuropharmacology 25(10) , 1161-6, (1986)] |
|
The memory-enhancing effects of the piracetam-like nootropic...
1989-05-01 [Behav. Brain Res. 33(1) , 79-82, (1989)] |
|
Gas chromatographic assay of pramiracetam in human plasma us...
[J. Chromatogr. A. 274 , 346-9, (1983)] |
|
Pramiracetam and epileptic after-discharges in young rats af...
1989-04-01 [Act. Nerv. Super. (Praha.) 31(1) , 68-9, (1989)] |
|
Pharmacokinetics of oral pramiracetam in normal volunteers.
1985-01-01 [J. Clin. Pharmacol. 25(4) , 291-5, (1985)] |