Validation of a liquid chromatographic/tandem mass spectrometric method for the determination of scopolamine butylbromide in human plasma: application of the method to a bioequivalence study.
Josélia Larger Manfio, Mauricio Bedim Dos Santos, Wagner Alex Jann Favreto, Fabiane Ines Hoffmann, Adriana Cristina Mertin
Index: J. AOAC Int. 92(5) , 1366-72, (2009)
Full Text: HTML
Abstract
A sensitive and specific LC/MS/MS method was developed and validated for the determination of scopolamine butylbromide in human plasma. Scopolamine butylbromide and propanolol (internal standard) were extracted from the plasma by liquid-liquid extraction with dichloromethane as the extraction solvent and separated on a C18 analytical column (50 x 4.6 mm id) maintained at 40 degrees C. The analytes were eluted at a constant flow rate of 0.45 mL/min; the mobile phase consisted of acetonitrile and a buffer of 5 mM ammonium acetate and 0.1% formic acid (60 + 40, v/v). The mass spectrometer, equipped with an electrospray source in the positive ionization mode, was set up in the multiple-reaction monitoring mode to monitor the transitions m/z 360.6 > 102.5 (scopolamine butylbromide) and m/z 259.7 > 115.6 (propanolol). The chromatographic separation was obtained within 2.0 min, and the responses were linear over the concentration range of 0.10-40.00 ng/mL. The mean extraction recoveries of scopolamine butylbromide and propanolol from plasma were 69.00 and 80.76%, respectively. Method validation parameters, such as specificity, linearity, precision, accuracy, and stability, were within the acceptable range. Moreover, when the proposed method was successfully applied to a pharmacokinetic study of healthy human volunteers, the results showed that the two scopolamine butylbromide formulations tested are not bioequivalent in rate and extent of absorption.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
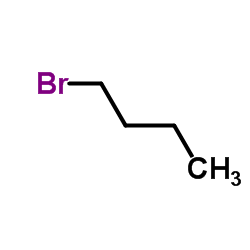 |
1-Bromobutane
CAS:109-65-9 |
C4H9Br |
|
Polymeric ionic liquid bucky gels as sorbent coatings for so...
2014-05-30 [J. Chromatogr. A. 1344 , 15-22, (2014)] |
|
A novel amino acid analysis method using derivatization of m...
2015-03-21 [Analyst 140(6) , 1965-73, (2015)] |
|
One-pot synthesis of 2,4,5-trisubstituted imidazoles using [...
2013-01-01 [Prep Biochem Biotechnol. 43(2) , 189-96, (2013)] |
|
Invoking pairwise interactions in water-promoted Diels-Alder...
2014-10-06 [ChemPhysChem 15(14) , 3067-77, (2014)] |
|
Differences in crystallization of two LinB variants from Sph...
2013-03-01 [Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 69(Pt 3) , 284-7, (2013)] |