| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Methanol
CAS:67-56-1 |
|
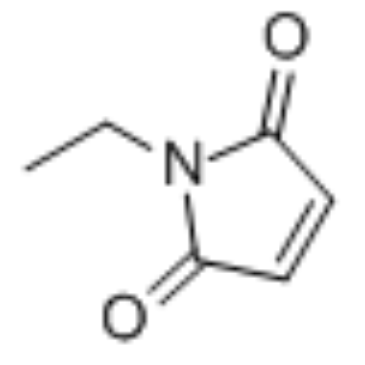 |
N-ethylmaleimide
CAS:128-53-0 |
|
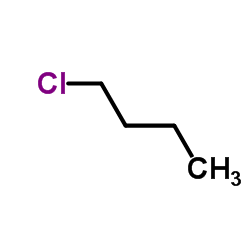 |
1-Chlorobutane
CAS:109-69-3 |
|
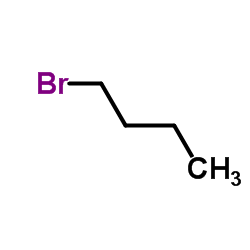 |
1-Bromobutane
CAS:109-65-9 |
|
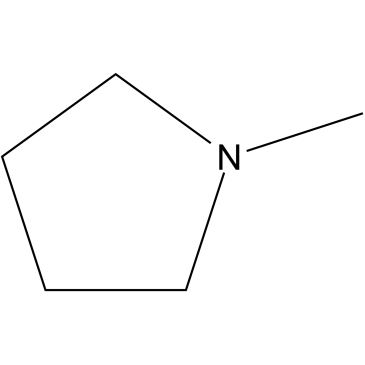 |
1-Methylpyrrolidine
CAS:120-94-5 |
|
 |
methylimidazole
CAS:616-47-7 |
|
 |
Butyl iodide
CAS:542-69-8 |