Organic & Biomolecular Chemistry
2009-05-07
Synthesis of chromones and 4-hydroxyquinolines based on uncatalyzed condensations of 1-methoxy-1,3-bis(trimethylsilyloxy)-1,3-butadiene with 2-alkoxy- and 2-nitrobenzoyl chlorides and related reactions.
Thomas Rahn, Bettina Appel, Wolfgang Baumann, Haijun Jiao, Armin Börner, Christine Fischer, Peter Langer
Index: Org. Biomol. Chem. 7(9) , 1931-8, (2009)
Full Text: HTML
Abstract
The reaction of 1-methoxy-1,3-bis(trimethylsilyloxy)-1,3-butadiene with 2-methoxybenzoyl chlorides afforded 3,5-diketoesters which were transformed, by treatment with boron tribromide, into functionalized 2-hydroxychroman-4-ones or chromones. The reaction of 1-methoxy-1,3-bis(trimethylsilyloxy)-1,3-butadiene with 2-nitrobenzoyl chlorides and subsequent reduction of the nitro group afforded functionalized 4-hydroxyquinolines. Their tautomeric equilibrium was studied by NMR spectroscopy and by computational methods.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
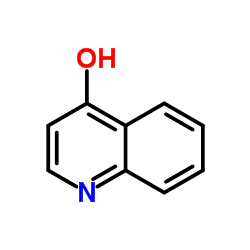 |
4-quinolone
CAS:611-36-9 |
C9H7NO |
Related Articles:
More...
|
Synergistic antidepressant-like effect of ferulic acid in co...
2015-12-01 [Metab. Brain Dis. 30 , 1505-14, (2015)] |
|
Evolution from a natural flavones nucleus to obtain 2-(4-Pro...
2011-08-25 [J. Med. Chem. 54 , 5722-36, (2011)] |
|
Antioxidant properties of 4-quinolones and structurally rela...
2012-01-15 [Bioorg. Med. Chem. 20 , 809-18, (2012)] |
|
5-(2-Aminopropyl)indole (5-IT): a psychoactive substance use...
2014-01-01 [Drug Test. Anal. 6(7-8) , 607-13, (2014)] |
|
Selective formation of glycosidic linkages of N-unsubstitute...
2010-04-19 [Carbohydr. Res. 345(6) , 768-79, (2010)] |