| Structure | Name/CAS No. | Articles |
|---|---|---|
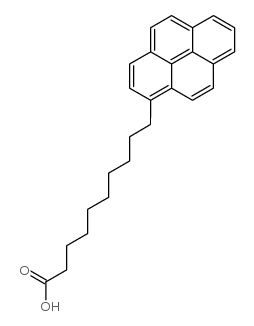 |
1-Pyrenedecanoic acid
CAS:64701-47-9 |
|
 |
Lauryl aldehyde
CAS:112-54-9 |
|
 |
Alcohol dehydrogenase
CAS:9031-72-5 |
|
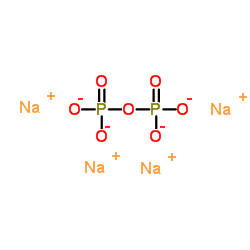 |
Tetrasodium pyrophosphate
CAS:7722-88-5 |