Chemical Communications
2011-02-07
A powerful synergistic effect for highly efficient diastereo- and enantioselective phase-transfer catalyzed conjugate additions.
Ming-Qing Hua, Lian Wang, Han-Feng Cui, Jing Nie, Xiao-Ling Zhang, Jun-An Ma
Index: Chem. Commun. (Camb.) 47(5) , 1631-3, (2011)
Full Text: HTML
Abstract
An efficient, catalytic, diastereo- and enantioselective conjugate addition of N-(diphenylmethylene)glycine tert-butyl ester to β-aryl substituted enones was realized in the presence of 1 mol% of newly desired dinuclear N-spiro-ammonium salts, affording functionalized α-amino acid derivatives in 57-98% yields with high diastereoselectivity (up to 99:1 dr) and enantioselectivity (up to 96.5:3.5 er).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
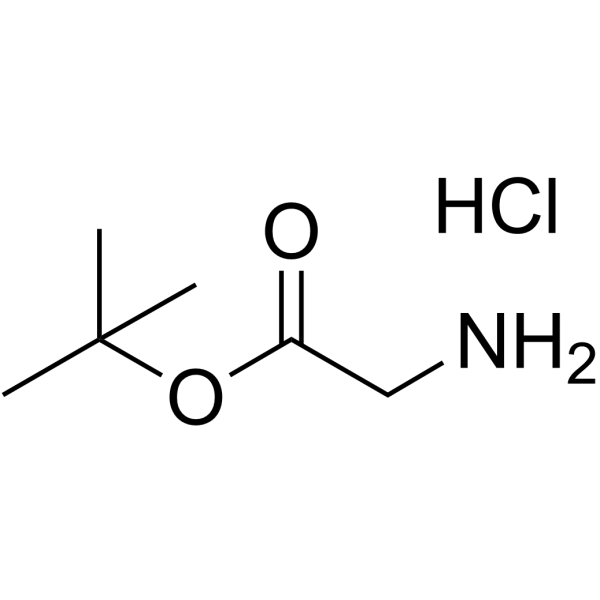 |
H-Gly-OtBu·HCl
CAS:27532-96-3 |
C6H14ClNO2 |
Related Articles:
More...
|
Inhibition of angiogenesis by antioxidant micelles.
2015-03-11 [Adv. Healthc. Mater. 4(4) , 569-75, (2015)] |
|
Synthesis and characterization of more potent analogues of h...
1998-09-29 [Biochemistry 37(39) , 13507-15, (1998)] |
|
Chiral phosphine-free Pd-mediated asymmetric allylation of p...
2001-10-18 [Org. Lett. 3(21) , 3329-31, (2001)] |
|
The amplification of the anticonvulsant effect of vinyl GABA...
1984-01-01 [Arzneimittelforschung 34(6) , 687-90, (1984)] |
|
Cinchona alkaloid-based polymer-bound phase-transfer catalys...
2005-01-01 [Mol. Divers. 9(4) , 277-90, (2005)] |