Analysis of the (Trimethylsilyl)propionic Acid-β(12-28) Peptide Binding Equilibrium with NMR Spectroscopy.
D A Jayawickrama, C K Larive
Index: Anal. Chem. 11th ed., 71 , 2117, (1999)
Full Text: HTML
Abstract
The binding of a small molecule, (trimethylsilyl)propionic acid (TSP), to a 17-residue peptide, β(12-28), is examined using (1)H NMR spectroscopy. β(12-28) (VHHQKLVFFAEDVGSNK) is a central fragment of the 40-42-residue Alzheimer's-associated Aβ peptide. This peptide has been previously shown to form soluble aggregates in low-pH aqueous solution. The TSP resonance is broadened appreciably in solutions containing relatively high concentrations (∼2 mM) of the peptide. The changes in TSP line width measured by titration of a peptide solution with TSP indicate a 1:1 binding stoichiometry. If the concentrations of both the peptide and TSP are reduced by 1 order of magnitude, the resonances of both species are sharp, suggesting that TSP binds predominately to the aggregated peptide. Nuclear Overhauser effect experiments indicate that the TSP interacts predominately with the side chains of the aliphatic peptide residues Leu(17) and Val(18). Pulsed-field gradient NMR measurements of TSP and peptide diffusion coefficients provide a more quantitative picture of the TSP-peptide binding equilibrium. The measured diffusion coefficients were used to calculate the fractions of the free and bound TSP. These results substantiate the conclusion that the stoichiometry of the TSP-peptide binding equilibrium is essentially 1:1 and further indicate anticooperative behavior in solutions containing an excess of TSP resulting in a dissociation of the peptide aggregates.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
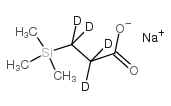 |
3-(Trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt
CAS:24493-21-8 |
C6H9D4NaO2Si |
|
A metabolomic study of adipose tissue in mice with a disrupt...
2015-07-01 [Mol. Biosyst. 11 , 1897-906, (2015)] |
|
Ethanol contamination of cerebrospinal fluid during standard...
2015-06-01 [Anal. Bioanal. Chem 407 , 4835-9, (2015)] |
|
Water Proton NMR for In Situ Detection of Insulin Aggregates...
2015-12-01 [J. Pharm. Sci. 104 , 4132-41, (2016)] |
|
The antitumor effect of formosanin C on HepG2 cell as reveal...
2014-09-05 [Chem. Biol. Interact. 220 , 193-9, (2014)] |
|
Metabolomics reveals the metabolic shifts following an inter...
2012-01-01 [Nutr. J. 11 , 88, (2012)] |