New chimeric antimalarials with 4-aminoquinoline moiety linked to a tetraoxane skeleton.
Igor Opsenica, Dejan Opsenica, Charlotte Anne Lanteri, Lalaine Anova, Wilbur K Milhous, Kirsten S Smith, Bogdan A Solaja
Index: J. Med. Chem. 51(19) , 6216-9, (2008)
Full Text: HTML
Abstract
The synthesis of the chimeric molecules consisting of two pharmacophores, tetraoxane and 7-chloro-4-aminoquinoline, is reported. The tetraoxanes 2, 4, and 8 show relatively potent in vitro antimalarial activities, with IC90 values for the Plasmodium falciparum strain W2 of 2.26, 12.44, and 10.74 nM, respectively. In addition, two compounds, 2 and 4, cured mice in a modified Thompson test for antimalarial blood stage activity, with a minimum curative dose of 80 mg/kg, a minimum active dose of 20 mg/kg/day, and a maximum tolerated dose of >960 mg/kg.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
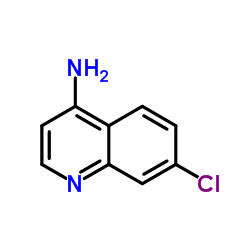 |
7-Chloro-4-quinolinamine
CAS:1198-40-9 |
C9H7ClN2 |
|
Overcoming drug resistance to heme-targeted antimalarials by...
2008-04-10 [J. Med. Chem. 51(7) , 1995-8, (2008)] |
|
An ELISA test for detecting chloroquine in urine.
1988-01-01 [Trans. R. Soc. Trop. Med. Hyg. 82(2) , 216-20, (1988)] |
|
Synthesis and comparison of antiplasmodial activity of (+), ...
2012-10-01 [Bioorg. Med. Chem. 20(19) , 5980-5, (2012)] |
|
Chloroquine metabolism in man: urinary excretion of 7-chloro...
[J. Chromatogr. A. 345(1) , 209-14, (1985)] |
|
Synthesis, antimalarial activity, and cellular toxicity of n...
2010-09-15 [Bioorg. Med. Chem. 18(18) , 6625-33, (2010)] |