Journal of Medicinal Chemistry
2008-04-10
Overcoming drug resistance to heme-targeted antimalarials by systematic side chain variation of 7-chloro-4-aminoquinolines.
Kimberly Yearick, Kekeli Ekoue-Kovi, Daniel P Iwaniuk, Jayakumar K Natarajan, John Alumasa, Angel C de Dios, Paul D Roepe, Christian Wolf
Index: J. Med. Chem. 51(7) , 1995-8, (2008)
Full Text: HTML
Abstract
Systematic variation of the branching and basicity of the side chain of chloroquine yielded a series of new 7-chloro-4-aminoquinoline derivatives exhibiting high in vitro activity against four different strains of P. falciparum. Many of the compounds tested showed excellent potency against chloroquine sensitive and resistant strains. In particular 4b, 5a, 5b, 5d, 17a, and 17b were found to be significantly more potent than chloroquine against the resistant strains Dd2 and FCB.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
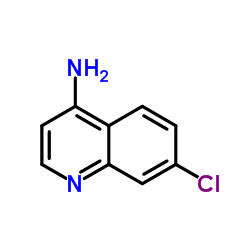 |
7-Chloro-4-quinolinamine
CAS:1198-40-9 |
C9H7ClN2 |
Related Articles:
More...
|
New chimeric antimalarials with 4-aminoquinoline moiety link...
2008-10-09 [J. Med. Chem. 51(19) , 6216-9, (2008)] |
|
An ELISA test for detecting chloroquine in urine.
1988-01-01 [Trans. R. Soc. Trop. Med. Hyg. 82(2) , 216-20, (1988)] |
|
Synthesis and comparison of antiplasmodial activity of (+), ...
2012-10-01 [Bioorg. Med. Chem. 20(19) , 5980-5, (2012)] |
|
Chloroquine metabolism in man: urinary excretion of 7-chloro...
[J. Chromatogr. A. 345(1) , 209-14, (1985)] |
|
Synthesis, antimalarial activity, and cellular toxicity of n...
2010-09-15 [Bioorg. Med. Chem. 18(18) , 6625-33, (2010)] |