Study of interaction of proton transfer probe 1-hydroxy-2-naphthaldehyde with serum albumins: a spectroscopic study.
Rupashree Balia Singh, Subrata Mahanta, Nikhil Guchhait
Index: J. Photochem. Photobiol. B, Biol. 91(1) , 1-8, (2008)
Full Text: HTML
Abstract
In the present work, we have studied the interaction of proton transfer probe 1-hydroxy-2-naphthaldehyde (HN12) with Human Serum Albumin (HSA) and Bovine Serum Albumin (BSA) by steady state absorption and emission spectroscopy combined with time resolved fluorescence measurements. The measured binding constant (K) and free energy change (DeltaG) indicate a stronger affinity of HN12 molecule for HSA than BSA. Steady state anisotropy, excitation anisotropy and fluorescence resonance energy transfer (FRET) studies indicate that the probe molecule resides at the hydrophobic site of the protein environment.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
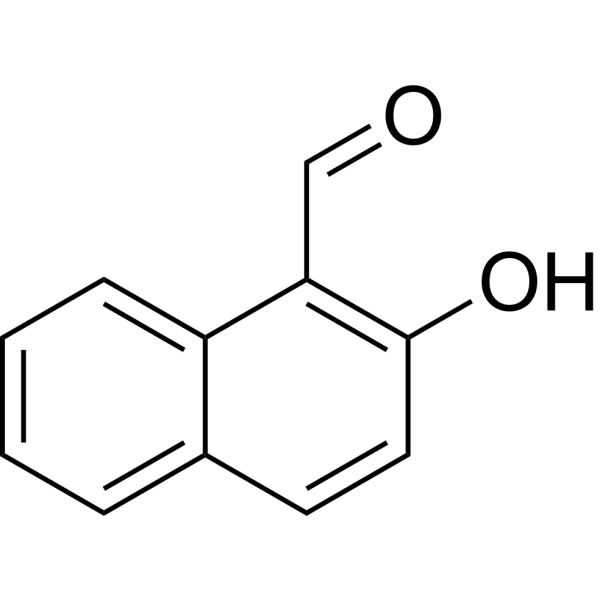 |
2-Hydroxy-naphthaldehyde
CAS:708-06-5 |
C11H8O2 |
|
Synthesis, spectroscopic, coordination and biological activi...
2015-10-05 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 149 , 771-87, (2015)] |
|
Schiff bases attached L-glutamine and L-asparagine: first in...
2014-06-01 [Artif. Cells. Nanomed. Biotechnol. 42(3) , 199-204, (2014)] |
|
Inhibition of ER stress-associated IRE-1/XBP-1 pathway reduc...
2014-06-01 [J. Clin. Invest. 124(6) , 2585-98, (2014)] |
|
Syntheses, characterization, biological activities and photo...
2011-10-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 81(1) , 570-7, (2011)] |
|
Naphthalene based colorimetric sensor for bioactive anions: ...
2013-03-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 105 , 477-82, (2013)] |