Nucleic Acids Symposium Series
1980-01-01
Dibutyltin oxide--phenyl isocyanate system for regioselective phenylcarbamoylation of the hydroxy-groups of ribonucleosides.
Y Ishido, I Hirao, K Itoh, K Tamaki, Y Araki
Index: Nucleic Acids Symp. Ser. (8) , s7-8, (1980)
Full Text: HTML
Abstract
For partial phenylcarbamoylation of the hydroxy-groups of ribonucleosides, dibutyltin oxide--phenyl isocyanate system was found to be surperior to the bis(tributyltin) oxide--phenyl isocyanate system from the standpoint of reaction procedures including isolation of the products; the reaction was proved to occur with similar regioselectivity and to give the corresponding 5'-, 3'-, and 2'-O-phenylcarbamoyl derivatives in good yields, respectively, due to the conditions used.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
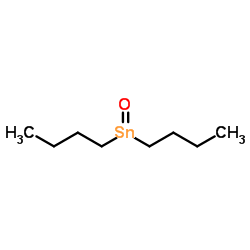 |
Dibutyltin oxide
CAS:818-08-6 |
C8H18OSn |
Related Articles:
More...
|
Copolyesters made from 1,4-butanediol, sebacic acid, and D-g...
2015-03-09 [Biomacromolecules 16(3) , 868-79, (2015)] |
|
Hydrogen-bond-mediated self-assembly of 26-membered diaza te...
2011-10-21 [J. Org. Chem. 76(20) , 8223-31, (2011)] |
|
[Preparation and spectroscopic studies of diorganotin(IV) co...
2000-01-01 [Acta Pharm. Hung. 70(3-6) , 119-30, (2000)] |
|
Synthesis of 2- and 4-nitrophenyl beta-glycosides of beta-(1...
1995-11-22 [Carbohydr. Res. 277(2) , 231-44, (1995)] |
|
A mild and selective method for cleavage of O-acetyl groups ...
2002-10-11 [Carbohydr. Res. 337(19) , 1763-7, (2002)] |